Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure
- PMID: 15932947
- PMCID: PMC1150816
- DOI: 10.1073/pnas.0500169102
Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure
Abstract
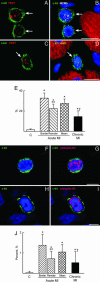
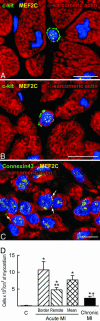
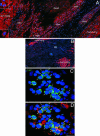
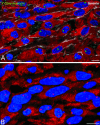
In this study, we tested whether the human heart possesses a cardiac stem cell (CSC) pool that promotes regeneration after infarction. For this purpose, CSC growth and senescence were measured in 20 hearts with acute infarcts, 20 hearts with end-stage postinfarction cardiomyopathy, and 12 control hearts. CSC number increased markedly in acute and, to a lesser extent, in chronic infarcts. CSC growth correlated with the increase in telomerase-competent dividing CSCs from 1.5% in controls to 28% in acute infarcts and 14% in chronic infarcts. The CSC mitotic index increased 29-fold in acute and 14-fold in chronic infarcts. CSCs committed to the myocyte, smooth muscle, and endothelial cell lineages increased approximately 85-fold in acute infarcts and approximately 25-fold in chronic infarcts. However, p16(INK4a)-p53-positive senescent CSCs also increased and were 10%, 18%, and 40% in controls, acute infarcts, and chronic infarcts, respectively. Old CSCs had short telomeres and apoptosis involved 0.3%, 3.8%, and 9.6% of CSCs in controls, acute infarcts, and chronic infarcts, respectively. These variables reduced the number of functionally competent CSCs from approximately 26,000/cm3 of viable myocardium in acute to approximately 7,000/cm3 in chronic infarcts, respectively. In seven acute infarcts, foci of spontaneous myocardial regeneration that did not involve cell fusion were identified. In conclusion, the human heart possesses a CSC compartment, and CSC activation occurs in response to ischemic injury. The loss of functionally competent CSCs in chronic ischemic cardiomyopathy may underlie the progressive functional deterioration and the onset of terminal failure.
Figures


References
-
- Beltrami, A. P., Urbanek, K., Kajstura, J., Yan, S. M., Finato, N., Bussani, R., Nadal-Ginard, B., Silvestri, F., Leri, A., Beltrami, C. A., et al. (2001) N. Engl. J. Med. 344, 1750-1757. - PubMed
-
- Quaini, F., Urbanek, K., Beltrami, A. P., Finato, N., Beltrami, C. A., Nadal-Ginard, B., Kajstura, J., Leri, A. & Anversa, P. (2002) N. Engl. J. Med. 346, 5-15. - PubMed
-
- Beltrami, A. P., Barlucchi, L., Torella, D., Baker, M., Limana, F., Chimenti, S., Kasahara, H., Rota, M., Musso, E., Urbanek, K., et al. (2003) Cell 114, 763-776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL66923/HL/NHLBI NIH HHS/United States
- R37 HL055757/HL/NHLBI NIH HHS/United States
- R01 AG017042/AG/NIA NIH HHS/United States
- HL76794/HL/NHLBI NIH HHS/United States
- R01 HL076794/HL/NHLBI NIH HHS/United States
- HL075480/HL/NHLBI NIH HHS/United States
- AG023071/AG/NIA NIH HHS/United States
- HL38132/HL/NHLBI NIH HHS/United States
- R01 HL055757/HL/NHLBI NIH HHS/United States
- R01 HL038132/HL/NHLBI NIH HHS/United States
- HL081737/HL/NHLBI NIH HHS/United States
- R01 HL075480/HL/NHLBI NIH HHS/United States
- R01 HL070897/HL/NHLBI NIH HHS/United States
- HL65577/HL/NHLBI NIH HHS/United States
- AG15756/AG/NIA NIH HHS/United States
- HL70897/HL/NHLBI NIH HHS/United States
- HL68088/HL/NHLBI NIH HHS/United States
- AG17042/AG/NIA NIH HHS/United States
- HL55757/HL/NHLBI NIH HHS/United States
- HL65573/HL/NHLBI NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- P01 AG023071/AG/NIA NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
- R01 HL068088/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

