ASCIZ regulates lesion-specific Rad51 focus formation and apoptosis after methylating DNA damage
- PMID: 15933716
- PMCID: PMC1173145
- DOI: 10.1038/sj.emboj.7600704
ASCIZ regulates lesion-specific Rad51 focus formation and apoptosis after methylating DNA damage
Abstract
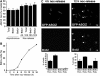
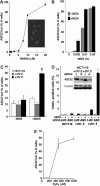
Nuclear Rad51 focus formation is required for homology-directed repair of DNA double-strand breaks (DSBs), but its regulation in response to non-DSB lesions is poorly understood. Here we report a novel human SQ/TQ cluster domain-containing protein termed ASCIZ that forms Rad51-containing foci in response to base-modifying DNA methylating agents but not in response to DSB-inducing agents. ASCIZ foci seem to form prior to Rad51 recruitment, and an ASCIZ core domain can concentrate Rad51 in focus-like structures independently of DNA damage. ASCIZ depletion dramatically increases apoptosis after methylating DNA damage and impairs Rad51 focus formation in response to methylating agents but not after ionizing radiation. ASCIZ focus formation and increased apoptosis in ASCIZ-depleted cells depend on the mismatch repair protein MLH1. Interestingly, ASCIZ foci form efficiently during G1 phase, when sister chromatids are unavailable as recombination templates. We propose that ASCIZ acts as a lesion-specific focus scaffold in a Rad51-dependent pathway that resolves cytotoxic repair intermediates, most likely single-stranded DNA gaps, resulting from MLH1-dependent processing of base lesions.
Figures





Similar articles
-
Mismatch repair proteins are activators of toxic responses to chromium-DNA damage.Mol Cell Biol. 2005 May;25(9):3596-607. doi: 10.1128/MCB.25.9.3596-3607.2005. Mol Cell Biol. 2005. PMID: 15831465 Free PMC article.
-
Signalling cell cycle arrest and cell death through the MMR System.Carcinogenesis. 2006 Apr;27(4):682-92. doi: 10.1093/carcin/bgi298. Epub 2005 Dec 6. Carcinogenesis. 2006. PMID: 16332722 Review.
-
Mechanisms of DNA double strand break repair and chromosome aberration formation.Cytogenet Genome Res. 2004;104(1-4):14-20. doi: 10.1159/000077461. Cytogenet Genome Res. 2004. PMID: 15162010 Review.
-
A breathtaking phenotype: unexpected roles of the DNA base damage response protein ASCIZ as a key regulator of early lung development.Cell Cycle. 2011 Apr 15;10(8):1222-4. doi: 10.4161/cc.10.8.15336. Epub 2011 Apr 15. Cell Cycle. 2011. PMID: 21415597
-
BRCA2-dependent and independent formation of RAD51 nuclear foci.Oncogene. 2003 Feb 27;22(8):1115-23. doi: 10.1038/sj.onc.1206263. Oncogene. 2003. PMID: 12606939
Cited by
-
Multivalency regulates activity in an intrinsically disordered transcription factor.Elife. 2018 May 1;7:e36258. doi: 10.7554/eLife.36258. Elife. 2018. PMID: 29714690 Free PMC article.
-
ATMIN is required for maintenance of genomic stability and suppression of B cell lymphoma.Cancer Cell. 2011 May 17;19(5):587-600. doi: 10.1016/j.ccr.2011.03.022. Cancer Cell. 2011. PMID: 21575860 Free PMC article.
-
The Zinc-finger protein ASCIZ regulates B cell development via DYNLL1 and Bim.J Exp Med. 2012 Aug 27;209(9):1629-39. doi: 10.1084/jem.20120785. Epub 2012 Aug 13. J Exp Med. 2012. PMID: 22891272 Free PMC article.
-
Dual functions of ASCIZ in the DNA base damage response and pulmonary organogenesis.PLoS Genet. 2010 Oct 21;6(10):e1001170. doi: 10.1371/journal.pgen.1001170. PLoS Genet. 2010. PMID: 20975950 Free PMC article.
-
Accidental Encounter of Repair Intermediates in Alkylated DNA May Lead to Double-Strand Breaks in Resting Cells.Int J Mol Sci. 2024 Jul 26;25(15):8192. doi: 10.3390/ijms25158192. Int J Mol Sci. 2024. PMID: 39125763 Free PMC article. Review.
References
-
- Adamson AW, Kim WJ, Shangary S, Baskaran R, Brown KD (2002) ATM is activated in response to N-methyl-N′-nitro-N-nitrosoguanidine-induced DNA alkylation. J Biol Chem 277: 38222–38229 - PubMed
-
- Barnes DE, Lindahl T (2004) Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet 38: 445–476 - PubMed
-
- Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM (2001) WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res 61: 8068–8073 - PubMed
-
- Bishop DK, Ear U, Bhattacharyya A, Calderone C, Beckett M, Weichselbaum RR, Shinohara A (1998) Xrcc3 is required for assembly of Rad51 complexes in vivo. J Biol Chem 273: 21482–21488 - PubMed
-
- Chen CF, Chen PL, Zhong Q, Sharp ZD, Lee WH (1999) Expression of BRC repeats in breast cancer cells disrupts the BRCA2-Rad51 complex and leads to radiation hypersensitivity and loss of G(2)/M checkpoint control. J Biol Chem 274: 32931–32935 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

