Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching
- PMID: 15934135
- PMCID: PMC391066
- DOI: 10.1038/sj.emboj.7600157
Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching
Retraction in
-
Retraction: 'Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching'.EMBO J. 2014 Dec 1;33(23):2880. doi: 10.15252/embj.201470100. EMBO J. 2014. PMID: 25452583 Free PMC article. No abstract available.
Abstract
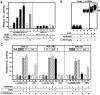
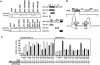
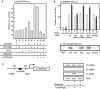
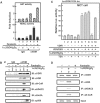
Vitamin D receptor (VDR) is essential for ligand-induced gene repression of 25(OH)D3 1alpha-hydroxylase (1alpha(OH)ase) in mammalian kidney, while this gene expression is activated by protein kinase A (PKA) signaling downstream of the parathyroid hormone action. The mapped negative vitamin D response element (1alphanVDRE) in the human 1alpha(OH)ase gene promoter (around 530 bp) was distinct from those of the reported DR3-like nVDREs, composed of two E-box-like motifs. Unlike the reported nVDREs, no direct binding of VDR/RXR heterodimer to 1alphanVDRE was detected. A bHLH-type factor, designated VDIR, was identified as a direct sequence-specific activator of 1nVDRE. The transactivation function of VDIR was further potentiated by activated-PKA signaling through phosphorylation of serine residues in the transactivation domains, with the recruitment of a p300 histone acetyltransferase co-activator. The ligand-dependent association of VDR/RXR heterodimer with VDIR bound to 1alphanVDRE caused the dissociation of p300 co-activators from VDIR, and the association of HDAC co-repressor complex components resulting in ligand-induced transrepression. Thus, the present study deciphers a novel mechanism of ligand-induced transrepression by nuclear receptor via co-regulator switching.
Figures


Similar articles
-
1Alpha,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter.Mol Endocrinol. 2007 Feb;21(2):334-42. doi: 10.1210/me.2006-0231. Epub 2006 Nov 9. Mol Endocrinol. 2007. PMID: 17095575
-
1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts.J Bone Miner Res. 2005 Feb;20(2):305-17. doi: 10.1359/JBMR.041112. Epub 2004 Nov 16. J Bone Miner Res. 2005. PMID: 15647825
-
Liganded Vitamin D Receptor Through Its Interacting Repressor Inhibits the Expression of Type I Collagen α1.DNA Cell Biol. 2016 Sep;35(9):498-505. doi: 10.1089/dna.2016.3367. Epub 2016 Jun 28. DNA Cell Biol. 2016. PMID: 27351590
-
The vitamin D hormone and its nuclear receptor: molecular actions and disease states.J Endocrinol. 1997 Sep;154 Suppl:S57-73. J Endocrinol. 1997. PMID: 9379138 Review.
-
Relationship of structure and function of DNA-binding domain in vitamin D receptor.Molecules. 2015 Jul 7;20(7):12389-99. doi: 10.3390/molecules200712389. Molecules. 2015. PMID: 26198224 Free PMC article. Review.
Cited by
-
Equivalent anticancer activities of dietary vitamin D and calcitriol in an animal model of breast cancer: importance of mammary CYP27B1 for treatment and prevention.J Steroid Biochem Mol Biol. 2013 Jul;136:289-95. doi: 10.1016/j.jsbmb.2012.08.005. Epub 2012 Aug 23. J Steroid Biochem Mol Biol. 2013. PMID: 22939886 Free PMC article. Review.
-
Genomic determinants of gene regulation by 1,25-dihydroxyvitamin D3 during osteoblast-lineage cell differentiation.J Biol Chem. 2014 Jul 11;289(28):19539-54. doi: 10.1074/jbc.M114.578104. Epub 2014 Jun 2. J Biol Chem. 2014. PMID: 24891508 Free PMC article.
-
Nuclear receptors in bone physiology and diseases.Physiol Rev. 2013 Apr;93(2):481-523. doi: 10.1152/physrev.00008.2012. Physiol Rev. 2013. PMID: 23589826 Free PMC article. Review.
-
IGFBP-3 Interacts with the Vitamin D Receptor in Insulin Signaling Associated with Obesity in Visceral Adipose Tissue.Int J Mol Sci. 2017 Nov 7;18(11):2349. doi: 10.3390/ijms18112349. Int J Mol Sci. 2017. PMID: 29112142 Free PMC article.
-
25-Hydroxyvitamin D3 is a natural chemopreventive agent against carcinogen induced precancerous lesions in mouse mammary gland organ culture.Breast Cancer Res Treat. 2009 Jan;113(1):31-41. doi: 10.1007/s10549-008-9900-0. Epub 2008 Jan 20. Breast Cancer Res Treat. 2009. PMID: 18205042 Free PMC article.
References
-
- Beato M, Heerrlich P, Chambon P (1995) Steroid hormone receptors: many actors in search of a plot. Cell 83: 851–857 - PubMed
-
- Beckmann H, Su LK, Kadesch T (1990) TFE3: a helix–loop–helix protein that activates transcription through the immunoglobulin enhancer muE3 motif. Genes Dev 4: 167–179 - PubMed
-
- Belandia B, Parker MG (2003) Nuclear receptors: a rendezvous for chromatin remodeling factors. Cell 114: 277–280 - PubMed
-
- Chambon P (1996) A decade of molecular biology of retinoic acid receptors. FASEB J 10: 940–954 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

