Chlamydophila pneumoniae induces expression of toll-like receptor 4 and release of TNF-alpha and MIP-2 via an NF-kappaB pathway in rat type II pneumocytes
- PMID: 15935092
- PMCID: PMC1180473
- DOI: 10.1186/1465-9921-6-51
Chlamydophila pneumoniae induces expression of toll-like receptor 4 and release of TNF-alpha and MIP-2 via an NF-kappaB pathway in rat type II pneumocytes
Abstract
Background: The role of alveolar type II cells in the regulation of innate and adaptive immunity is unclear. Toll-like receptors (TLRs) have been implicated in host defense. The purpose of the present study was to investigate whether Chlamydophila pneumoniae (I) alters the expression of TLR2 and/orTLR4 in type II cells in a (II) Rho-GTPase- and (III) NF-kappaB-dependent pathway, subsequently (IV) leading to the production of (IV) pro-inflammatory TNF-alpha and MIP-2.
Methods: Isolated rat type II pneumocytes were incubated with C. pneumoniae after pre-treatment with calcium chelator BAPTA-AM, inhibitors of NF-kappaB (parthenolide, SN50) or with a specific inhibitor of the Rho-GTPase (mevastatin). TLR2 and TLR4 mRNA expressions were analyzed by PCR. Activation of TLR4, Rac1, RhoA protein and NF-kappaB was determined by Western blotting and confocal laser scan microscopy (CLSM) and TNF-alpha and MIP-2 release by ELISA.
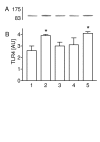
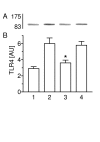
Results: Type II cells constitutively expressed TLR4 and TLR2 mRNA. A prominent induction of TLR4 but not TLR2 mRNA was detected after 2 hours of incubation with C. pneumoniae. The TLR4 protein expression reached a peak at 30 min, began to decrease within 1-2 hours and peaked again at 3 hours. Incubation of cells with heat-inactivated bacteria (56 degrees C for 30 min) significantly reduced the TLR4 expression. Treated bacteria with polymyxin B (2 mug/ml) did not alter TLR4 expression. C. pneumoniae-induced NF-kappaB activity was blocked by TLR4 blocking antibodies. TLR4 mRNA and protein expression were inhibited in the presence of BAPTA-AM, SN50 or parthenolide. TNF-alpha and MIP-2 release was increased in type II cells in response to C. pneumoniae, whereas BAPTA-AM, SN50 or parthenolide decreased the C. pneumoniae-induced TNF-alpha and MIP-2 release. Mevastatin inhibited C. pneumoniae-mediated Rac1, RhoA and TLR4 expression.
Conclusion: The TLR4 protein expression in rat type II cells is likely to be mediated by a heat-sensitive C. pneumoniae protein that induces a fast Ca2+-mediated NF-kappaB activity, necessary for maintenance of TLR4 expression and TNF-alpha and MIP-2 release through possibly Rac and Rho protein-dependent mechanism. These results indicate that type II pneumocytes play an important role in the innate pulmonary immune system and in inflammatory response mechanism of the alveolus.
Figures





Similar articles
-
Perfluorocarbons decrease Chlamydophila pneumoniae-mediated inflammatory responses of rat type II pneumocytes in vitro.Pediatr Res. 2006 Sep;60(3):264-9. doi: 10.1203/01.pdr.0000233033.82664.91. Epub 2006 Jul 20. Pediatr Res. 2006. PMID: 16857767
-
Triggering of toll-like receptors 2 and 4 by Aspergillus fumigatus conidia in immortalized human corneal epithelial cells to induce inflammatory cytokines.Chin Med J (Engl). 2008 Mar 5;121(5):450-4. Chin Med J (Engl). 2008. PMID: 18364120
-
Contact of Chlamydophila pneumoniae with type II cell triggers activation of calcium-mediated NF-kappa B pathway.Biochim Biophys Acta. 2005 Mar 22;1743(1-2):37-48. doi: 10.1016/j.bbamcr.2004.08.009. Biochim Biophys Acta. 2005. PMID: 15777838
-
Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility.Front Immunol. 2020 Jul 8;11:1455. doi: 10.3389/fimmu.2020.01455. eCollection 2020. Front Immunol. 2020. PMID: 32733481 Free PMC article. Review.
-
Bioactive Compounds and Probiotics Mitigate Mastitis by Targeting NF-κB Signaling Pathway.Biomolecules. 2024 Aug 15;14(8):1011. doi: 10.3390/biom14081011. Biomolecules. 2024. PMID: 39199398 Free PMC article. Review.
Cited by
-
A four-part guide to lung immunology: Invasion, inflammation, immunity, and intervention.Front Immunol. 2023 Mar 31;14:1119564. doi: 10.3389/fimmu.2023.1119564. eCollection 2023. Front Immunol. 2023. PMID: 37063828 Free PMC article. Review.
-
Role of primary human alveolar epithelial cells in host defense against Francisella tularensis infection.Infect Immun. 2007 Aug;75(8):3969-78. doi: 10.1128/IAI.00157-07. Epub 2007 May 14. Infect Immun. 2007. PMID: 17502386 Free PMC article.
-
The association between Chlamydia pneumoniae infection and prognosis in lung cancer patients: a prospective study.BMC Infect Dis. 2025 Jan 25;25(1):119. doi: 10.1186/s12879-025-10515-3. BMC Infect Dis. 2025. PMID: 39863864 Free PMC article.
-
Chlamydia pneumoniae-induced tumour necrosis factor alpha responses are lower in children with asthma compared with non-asthma.BMJ Open Respir Res. 2018 May 5;5(1):e000239. doi: 10.1136/bmjresp-2017-000239. eCollection 2018. BMJ Open Respir Res. 2018. PMID: 29755754 Free PMC article.
-
Mechanobiology of Pulmonary Diseases: A Review of Engineering Tools to Understand Lung Mechanotransduction.J Biomech Eng. 2021 Nov 1;143(11):110801. doi: 10.1115/1.4051118. J Biomech Eng. 2021. PMID: 33973005 Free PMC article. Review.
References
-
- Beaty CD, Grayston JT, Wang SP, Kuo CC, Reto CS, Martin TR. Chlamydia pneumoniae strain TWAR, infection in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;144:1408–1410. - PubMed
-
- Rupp J, Droemann D, Goldmann T, Zabel P, Solbach W, Vollmer E, Branscheid D, Dalhoff K, Maass M. Alveolar epithelial cells type II are major target cells for C. pneumoniae in chronic but not in acute respiratory infection. FEMS Immun Microbiol. 2004;41:197–203. doi: 10.1016/j.femsim.2004.03.004. - DOI - PubMed
-
- Droemann D, Goldmann T, Branscheid D, Clark R, Dalhoff K, Zabel P, Vollmer E. Toll-like receptor 2 is expressed by alveolar epithelial cells type II and macrophages in the human lung. Histochem Cell Biol. 2003;119:103–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

