Plasma membrane localization and function of TRPC1 is dependent on its interaction with beta-tubulin in retinal epithelium cells
- PMID: 15935109
- PMCID: PMC3619404
- DOI: 10.1017/S0952523805222058
Plasma membrane localization and function of TRPC1 is dependent on its interaction with beta-tubulin in retinal epithelium cells
Abstract
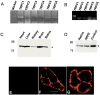
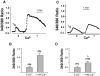
Mammalian homologues of the Drosophila canonical Transient Receptor Potential (TRPC) protein have been proposed to encode the store-operated Ca2+ influx (SOC) channel(s). This study examines the role of TRPC1 in the SOC mechanism of retinal cells. htrpc1 transcript was detected in bovine retinal and in human adult retinal pigment epithelial (ARPE) cells. Western blot analysis also confirmed the expression of TRPC1 protein in neuronal cells including retina and ARPE cells. To determine the role of TRPC1 protein in retinal cells, TRPC1 was recombinantly expressed in ARPE cells and changes in intracellular Ca2+ were analyzed. ARPE cells stably transfected with htrp1 cDNA displayed 2-fold higher Ca2+ influx with no significant increase in the basal influx. Consistent with this the overexpressed TRPC1 protein was localized in the plasma membrane region of ARPE cells. Interestingly, both bovine retinal tissues and ARPE cells showed that TRPC1 protein co-localizes and could be co-immunoprecipitated with beta-tubulin. Disruption of tubulin by colchicine significantly decreased both plasma membrane staining of the TRPC1 protein and Ca2+ influx in ARPE cells. These results suggest that TRPC1 channel protein is expressed in retinal cells, further, targeting/retention of the TRPC1 protein to the plasma membrane in retinal cells is mediated via its interaction with beta-tubulin.
Figures


Similar articles
-
Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3.J Biol Chem. 2005 Jun 3;280(22):21600-6. doi: 10.1074/jbc.C400492200. Epub 2005 Apr 17. J Biol Chem. 2005. PMID: 15834157
-
Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability.Am J Physiol Lung Cell Mol Physiol. 2004 Dec;287(6):L1303-13. doi: 10.1152/ajplung.00240.2004. Epub 2004 Sep 3. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 15347566
-
Basal calcium entry in retinal pigment epithelial cells is mediated by TRPC channels.Invest Ophthalmol Vis Sci. 2007 Dec;48(12):5767-72. doi: 10.1167/iovs.07-0412. Invest Ophthalmol Vis Sci. 2007. PMID: 18055830
-
Plasma membrane localization of TRPC channels: role of caveolar lipid rafts.Novartis Found Symp. 2004;258:63-70; discussion 70-4, 98-102, 263-6. Novartis Found Symp. 2004. PMID: 15104176 Review.
-
TRPC1: the link between functionally distinct store-operated calcium channels.Cell Calcium. 2007 Aug;42(2):213-23. doi: 10.1016/j.ceca.2007.01.013. Epub 2007 Mar 12. Cell Calcium. 2007. PMID: 17350680 Review.
Cited by
-
The odyssey of the TR(i)P journey to the cellular membrane.Front Cell Dev Biol. 2024 Jul 23;12:1414935. doi: 10.3389/fcell.2024.1414935. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39108834 Free PMC article. Review.
-
TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces.Cell Calcium. 2017 May;63:33-39. doi: 10.1016/j.ceca.2016.12.009. Epub 2016 Dec 30. Cell Calcium. 2017. PMID: 28089266 Free PMC article. Review.
-
Retinal TRP channels: Cell-type-specific regulators of retinal homeostasis and multimodal integration.Prog Retin Eye Res. 2023 Jan;92:101114. doi: 10.1016/j.preteyeres.2022.101114. Epub 2022 Sep 24. Prog Retin Eye Res. 2023. PMID: 36163161 Free PMC article. Review.
-
IKs response to protein kinase A-dependent KCNQ1 phosphorylation requires direct interaction with microtubules.Cardiovasc Res. 2008 Aug 1;79(3):427-35. doi: 10.1093/cvr/cvn085. Epub 2008 Apr 5. Cardiovasc Res. 2008. PMID: 18390900 Free PMC article.
-
Organization and function of TRPC channelosomes.Pflugers Arch. 2007 Nov;455(2):187-200. doi: 10.1007/s00424-007-0252-0. Epub 2007 May 8. Pflugers Arch. 2007. PMID: 17486362 Review.
References
-
- Berridge MD. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
-
- Crousillac S, LeRouge M, Rankin M, Gleason E. Immunolocalization of the TRPC channel subunits 1 and 4 in the chiken retina. Visual Neuroscience. 2003;20:453–463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

