Cellular basis for age-related changes in fracture repair
- PMID: 15936915
- PMCID: PMC2844440
- DOI: 10.1016/j.orthres.2005.04.003.1100230610
Cellular basis for age-related changes in fracture repair
Abstract
The goal of this work was to define cellular and molecular changes that occur during fracture healing as animals age. We compared the molecular, cellular, and histological progression of skeletal repair in juvenile (4 weeks old), middle-aged (6 months old), and elderly (18 months old) mice at 3, 5, 7, 10, 14, 21, 28, and 35 days post-fracture, using a non-stabilized tibia fracture model. Our histological and molecular analyses demonstrated that there was a sharp decline in fracture healing potential between juvenile and middle-aged animals, while a more subtle decrease in healing potential was apparent between middle-aged and elderly mice. By three days after fracture, chondrocytes expressing Collagen type II, and osteoblasts expressing osteocalcin, were present in calluses of juvenile, but not middle-aged or elderly, mice. At day 5 immature chondrocytes and osteoblasts were observed in calluses of middle-aged and elderly mice. While at this time, chondrocytes in juvenile mice were expressing Collagen type X (ColX) indicating that chondrocyte maturation was already underway. At day 7, chondrocytes expressing ColX were abundant in middle-aged mice while a small domain of ColX-positive chondrocytes were observed in elderly mice. Further, in juvenile and middle-aged mice, but not elderly mice, vascular invasion of the cartilage was underway by day 7. Juvenile mice had replaced nearly all of the cartilage by day 14, while cartilage was still present in the callus of middle-aged mice at day 21 and in elderly mice at day 28. In addition to these delays, histomorphometry revealed that elderly and middle-aged mice formed less bone than juveniles (p<0.001), while cartilage production was unaffected (p>0.22). Collectively, these data suggest that enhancing cell differentiation, improving osteoblast function, and accelerating endochondral ossification may significantly benefit the elderly.
Figures



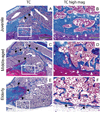

 indicates significant differences, p<0.05.
indicates significant differences, p<0.05.References
-
- Aho AJ. Electron microscopic and histologic studies on fracture repair in old and young rats. Acta Chir Scand Suppl. 1966;357:162–165. - PubMed
-
- Bak B, Andreassen TT. The effect of aging on fracture healing in the rat. Calcif Tissue Int. 1989;45:292–297. - PubMed
-
- Bellows CG, Pei W, Jia Y, et al. Proliferation, differentiation and self-renewal of osteoprogenitors in vertebral cell populations from aged and young female rats. Mech Ageing Dev. 2003;124:747–757. - PubMed
-
- Bergman RJ, Gazit D, Kahn AJ, et al. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11:568–577. - PubMed
-
- Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

