Brain maps, great and small: lessons from comparative studies of primate visual cortical organization
- PMID: 15937007
- PMCID: PMC1874231
- DOI: 10.1098/rstb.2005.1626
Brain maps, great and small: lessons from comparative studies of primate visual cortical organization
Abstract
In this paper, we review evidence from comparative studies of primate cortical organization, highlighting recent findings and hypotheses that may help us to understand the rules governing evolutionary changes of the cortical map and the process of formation of areas during development. We argue that clear unequivocal views of cortical areas and their homologies are more likely to emerge for "core" fields, including the primary sensory areas, which are specified early in development by precise molecular identification steps. In primates, the middle temporal area is probably one of these primordial cortical fields. Areas that form at progressively later stages of development correspond to progressively more recent evolutionary events, their development being less firmly anchored in molecular specification. The certainty with which areal boundaries can be delimited, and likely homologies can be assigned, becomes increasingly blurred in parallel with this evolutionary/developmental sequence. For example, while current concepts for the definition of cortical areas have been vindicated in allowing a clarification of the organization of the New World monkey "third tier" visual cortex (the third and dorsomedial areas, V3 and DM), our analyses suggest that more flexible mapping criteria may be needed to unravel the organization of higher-order visual association and polysensory areas.
Figures
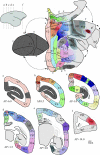
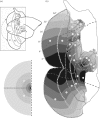
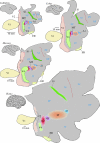

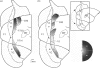


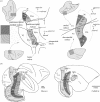
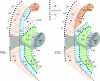


References
-
- Adams D.L, Horton J.C. Capricious expression of cortical columns in the primate brain. Nature Neurosci. 2003;6:113–114. - PubMed
-
- Allman J.M, Kaas J.H. A representation of the visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus) Brain Res. 1971;31:85–105. - PubMed
-
- Allman J.M, Kaas J.H. The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus) Brain Res. 1975;100:473–487. - PubMed
-
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb. Cortex. 2002;12:1202–1212. - PubMed
-
- Azzopardi P, Fallah M, Gross C.G, Rodman H.R. Response latencies of neurons in visual areas MT and MST of monkeys with striate cortex lesions. Neuropsychologia. 2003;41:1738–1756. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
