The chronoarchitecture of the cerebral cortex
- PMID: 15937010
- PMCID: PMC1569482
- DOI: 10.1098/rstb.2005.1627
The chronoarchitecture of the cerebral cortex
Abstract
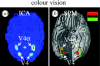
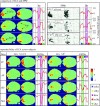
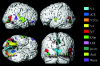
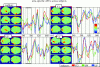
We review here a new approach to mapping the human cerebral cortex into distinct subdivisions. Unlike cytoarchitecture or traditional functional imaging, it does not rely on specific anatomical markers or functional hypotheses. Instead, we propose that the unique activity time course (ATC) of each cortical subdivision, elicited during natural conditions, acts as a temporal fingerprint that can be used to segregate cortical subdivisions, map their spatial extent, and reveal their functional and potentially anatomical connectivity. We argue that since the modular organisation of the brain and its connectivity evolved and developed in natural conditions, these are optimal for revealing its organisation. We review the concepts, methodology and first results of this approach, relying on data obtained with functional magnetic resonance imaging (fMRI) when volunteers viewed traditional stimuli or a James Bond movie. Independent component analysis (ICA) was used to identify voxels belonging to distinct functional subdivisions, based on their differential spatio-temporal fingerprints. Many more regions could be segregated during natural viewing, demonstrating that the complexity of natural stimuli leads to more differential responses in more functional modules. We demonstrate that, in a single experiment, a multitude of distinct regions can be identified across the whole brain, even within the visual cortex, including areas V1, V4 and V5. This differentiation is based entirely on the differential ATCs of different areas during natural viewing. Distinct areas can therefore be identified without any a priori hypothesis about their function or spatial location. The areas we identified corresponded anatomically across subjects, and their ATCs showed highly area-specific inter-subject correlations. Furthermore, natural conditions led to a significant de-correlation of interregional ATCs compared to rest, indicating an increase in regional specificity during natural conditions. In contrast, the correlation between ATCs of distant regions of known substantial anatomical connections increased and reflected their known anatomical connectivity pattern. We demonstrate this using the example of the language network involving Broca's and Wernicke's area and homologous areas in the two hemispheres. In conclusion, this new approach to brain mapping may not only serve to identify novel functional subdivisions, but to reveal their connectivity as well.
Figures










References
-
- Bartels A, Zeki S. The architecture of the colour centre in the human visual brain: new results and a review. Eur. J. Neurosci. 2000a;12:172–193. - PubMed
-
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000b;11:3829–3834. - PubMed
-
- Bartels A, Zeki S. The chronoarchitecture of the human brain—natural viewing conditions reveal a time-based anatomy of the brain. NeuroImage. 2004a;22:419–433. - PubMed
-
- Bartels A, Zeki S. The chronoarchitecture of the human brain: functional anatomy based on natural brain dynamics and the principle of functional independence. In: Frackowiak R, Friston K, Frith C, Dolan R, Zeki S, editors. Human brain function. 2nd edn. Academic Press; London: 2004b. pp. 201–229.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
