The importance of being agranular: a comparative account of visual and motor cortex
- PMID: 15937013
- PMCID: PMC1569485
- DOI: 10.1098/rstb.2005.1630
The importance of being agranular: a comparative account of visual and motor cortex
Abstract
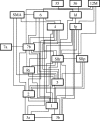
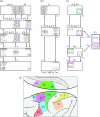
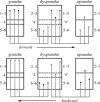
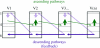
The agranular cortex is an important landmark-anatomically, as the architectural flag of mammalian motor cortex, and historically, as a spur to the development of theories of localization of function. But why, exactly, do agranularity and motor function go together? To address this question, it should be noted that not only does motor cortex lack granular layer four, it also has a relatively thinner layer three. Therefore, it is the two layers which principally constitute the ascending pathways through the sensory (granular) cortex that have regressed in motor cortex: simply stated, motor cortex does not engage in serial reprocessing of incoming sensory data. But why should a granular architecture not be demanded by the downstream relay of motor instructions through the motor cortex? The scant anatomical evidence available regarding laminar patterns suggests that the pathways from frontal and premotor areas to the primary motor cortex actually bear a greater resemblance to the descending, or feedback connections of sensory cortex that avoid the granular layer. The action of feedback connections is generally described as "modulatory" at a cellular level, or "selective" in terms of systems analysis. By contrast, ascending connections may be labelled "driving" or "instructive". Where the motor cortex uses driving inputs, they are most readily identified as sensory signals instructing the visual location of targets and the kinaesthetic state of the body. Visual signals may activate motor concepts, e.g. "mirror neurons", and the motor plan must select the appropriate muscles and forces to put the plan into action, if the decision to move is taken. This, perhaps, is why "driving" motor signals might be inappropriate-the optimal selection and its execution are conditional upon both kinaesthetic and motivational factors. The argument, summarized above, is constructed in honour of Korbinian Brodmann's centenary, and follows two of the fundamental principles of his school of thought: that uniformities in cortical structure, and development imply global conservation of some aspects of function, whereas regional variations in architecture can be used to chart the "organs" of the cortex, and perhaps to understand their functional differences.
Figures


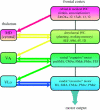
References
-
- Alexander G.E, Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Amassian V.E, Stewart M, Quirk G.J, Rosenthal J.L. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. - PubMed
-
- Andersen R.A, Asanuma A, Essick G, Siegel R.M. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J. Comp. Neurol. 1990;296:65–113. - PubMed
-
- Arikuni T, Watanabe K, Kubota K. Connections of area 8 with area 6 in the brain of the macaque monkey. J. Comp. Neurol. 1988;277:21–40. - PubMed
-
- Asanuma H, Arissian K. Experiments on functional role of peripheral input to motor cortex during voluntary movements in the monkey. J. Neurophysiol. 1984;52:212–227. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
