How human neuroblastoma cells make morphine
- PMID: 15937106
- PMCID: PMC1150847
- DOI: 10.1073/pnas.0503244102
How human neuroblastoma cells make morphine
Abstract
Recently, our laboratory demonstrated that human neuroblastoma cells (SH-SY5Y) are capable of synthesizing morphine, the major active metabolite of opium poppy. Now our experiments are further substantiated by extending the biochemical studies to the entire morphine pathway in this human cell line. L-[1,2,3-13C3]- and [ring-2',5',6'-2H3]dopa showed high isotopic enrichment and incorporation in both the isoquinoline and the benzyl moiety of the endogenous morphine. [2,2-2H2]Dopamine, however, was exclusively incorporated only into the isoquinoline moiety. Neither the trioxygenated (R,S)-[1,3-13C2]norcoclaurine, the precursor of morphine in the poppy plant, nor (R)-[1,3,4-2H3]norlaudanosoline showed incorporation into endogenous morphine. However, (S)-[1,3,4-2H3]norlaudanosoline furnished a good isotopic enrichment and the loss of a single deuterium atom at the C-9 position of the morphine molecule, indicating that the change of configuration from (S)- to (R)-reticuline occurs via the intermediacy of 1,2-dehydroreticuline. Additional feeding experiments with potential morphinan precursors demonstrated substantial incorporation of [7-2H]salutaridinol, but not 7-[7-2H]episalutaridinol, and [7-2H,N-C2H3]oripavine, and [6-2H]codeine into morphine. Human morphine biosynthesis involves at least 19 chemical steps. For the most part, it is a reflection of the biosynthesis in opium poppy; however, there is a fundamental difference in the formation of the key intermediate (S)-reticuline: it proceeds via the tetraoxygenated initial isoquinoline alkaloid (S)-norlaudanosoline, whereas the plant morphine biosynthesis proceeds via the trioxygenated (S)-norcoclaurine. Following the plant biosynthetic pathway, (S)-reticuline undergoes a change of configuration at C-1 during its transformation to salutaridinol and thebaine. From thebaine, there is a bifurcate pathway leading to morphine proceeding via codeine or oripavine, in both plants and mammals.
Figures

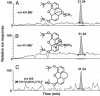
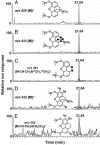


Similar articles
-
Mammalian morphine: de novo formation of morphine in human cells.Med Sci Monit. 2005 May;11(5):MS6-17. Epub 2005 Apr 28. Med Sci Monit. 2005. PMID: 15874902
-
Endogenous formation of morphine in human cells.Proc Natl Acad Sci U S A. 2004 Sep 28;101(39):14091-6. doi: 10.1073/pnas.0405430101. Epub 2004 Sep 21. Proc Natl Acad Sci U S A. 2004. PMID: 15383669 Free PMC article.
-
RNAi-mediated replacement of morphine with the nonnarcotic alkaloid reticuline in opium poppy.Nat Biotechnol. 2004 Dec;22(12):1559-66. doi: 10.1038/nbt1033. Epub 2004 Nov 14. Nat Biotechnol. 2004. PMID: 15543134
-
Evolution of morphine biosynthesis in opium poppy.Phytochemistry. 2009 Oct-Nov;70(15-16):1696-707. doi: 10.1016/j.phytochem.2009.07.006. Epub 2009 Aug 6. Phytochemistry. 2009. PMID: 19665152 Review.
-
Biosynthesis of the morphine alkaloids.Science. 1967 Jan 13;155(3759):170-3. doi: 10.1126/science.155.3759.170. Science. 1967. PMID: 5332945 Review.
Cited by
-
Dopamine, morphine, and nitric oxide: an evolutionary signaling triad.CNS Neurosci Ther. 2010 Jun;16(3):e124-37. doi: 10.1111/j.1755-5949.2009.00114.x. Epub 2009 Nov 13. CNS Neurosci Ther. 2010. PMID: 19912274 Free PMC article. Review.
-
Endogenous morphine in SH-SY5Y cells and the mouse cerebellum.PLoS One. 2008 Feb 20;3(2):e1641. doi: 10.1371/journal.pone.0001641. PLoS One. 2008. PMID: 18327293 Free PMC article.
-
Abnormal nociception and opiate sensitivity of STOP null mice exhibiting elevated levels of the endogenous alkaloid morphine.Mol Pain. 2010 Dec 20;6:96. doi: 10.1186/1744-8069-6-96. Mol Pain. 2010. PMID: 21172011 Free PMC article.
-
Urinary excretion of morphine and biosynthetic precursors in mice.Proc Natl Acad Sci U S A. 2010 May 4;107(18):8147-52. doi: 10.1073/pnas.1003423107. Epub 2010 Apr 26. Proc Natl Acad Sci U S A. 2010. PMID: 20421505 Free PMC article.
-
The presence of endogenous morphine signaling in animals.Neurochem Res. 2008 Oct;33(10):1933-9. doi: 10.1007/s11064-008-9674-0. Epub 2008 Sep 6. Neurochem Res. 2008. PMID: 18777209 Review.
References
-
- Hazum, E., Sabatka, J. J., Chang, K., Brent, D. A., Findlay, J. W. A. & Cuatrecasas, P. (1981) Science 213, 1010-1012. - PubMed
-
- Rowell, F. J., Spark, P. & Rich, C. G. (1982) J. Pharmacol. 77, 461P.
-
- Hofmann, U., Seefried, S., Schweizer, E., Ebner, T., Mikus, G. & Eichelbaum, M. (1999) J. Chromatogr. B. Biomed. Sci. Appl. 727, 81-88. - PubMed
-
- Spenser, I. D. (1968) in Comprehensive Biochemistry, eds. Florkin, M. & Stotz, E. H. (Elsevier, New York), Vol. 20, pp. 231-413.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

