Golgi targeting of human guanylate-binding protein-1 requires nucleotide binding, isoprenylation, and an IFN-gamma-inducible cofactor
- PMID: 15937107
- PMCID: PMC1150846
- DOI: 10.1073/pnas.0503227102
Golgi targeting of human guanylate-binding protein-1 requires nucleotide binding, isoprenylation, and an IFN-gamma-inducible cofactor
Abstract
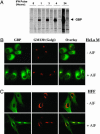
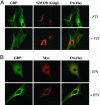
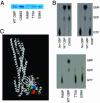
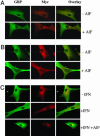
Human guanylate-binding protein-1 (hGBP-1) is a large GTPase, similar in structure to the dynamins. Like many smaller GTPases of the Ras/Rab family, it is farnesylated, suggesting it may dock into membranes and perhaps play a role in intracellular trafficking. To date, however, hGBP-1 has never been associated with a specific intracellular compartment. Here we present evidence that hGBP-1 can associate with the Golgi apparatus. Redistribution from the cytosol to the Golgi was observed by immunofluorescence and subcellular fractionation after aluminum fluoride treatment, suggesting that it occurs when hGBP-1 is in its GTP-bound state. Relocalization was blocked by a farnesyl transferase inhibitor. The C589S mutant of hGBP-1, which cannot be farnesylated, and the previously uncharacterized R48P mutant, which cannot bind GTP, both failed to localize to the Golgi. These two mutants had a dominant-negative effect, preventing endogenous wild-type hGBP-1 from efficiently redistributing after aluminum fluoride treatment. Furthermore, hGBP-1 requires another IFN-gamma-induced factor to be targeted to the Golgi, because constitutively expressed hGBP-1 remained cytosolic in cells treated with aluminum fluoride unless the cells were preincubated with IFN-gamma. Finally, two nonhydrolyzing mutants of hGBP-1, corresponding to active mutants of Ras family proteins, failed to constitutively associate with the Golgi; we propose three possible explanations for this surprising result.
Figures

Similar articles
-
Understanding the lower GMP formation in large GTPase hGBP-2 and role of its individual domains in regulation of GTP hydrolysis.FEBS J. 2019 Oct;286(20):4103-4121. doi: 10.1111/febs.14957. Epub 2019 Jul 1. FEBS J. 2019. PMID: 31199074
-
Interferon gamma-induced human guanylate binding protein 1 inhibits mammary tumor growth in mice.Mol Med. 2010 May-Jun;16(5-6):177-87. doi: 10.2119/molmed.2009.00172. Epub 2010 Feb 5. Mol Med. 2010. PMID: 20454519 Free PMC article.
-
Catalytic activity of human guanylate-binding protein 1 coupled to the release of structural restraints imposed by the C-terminal domain.FEBS J. 2021 Jan;288(2):582-599. doi: 10.1111/febs.15348. Epub 2020 May 19. FEBS J. 2021. PMID: 32352209
-
Rab GTPases: master regulators of membrane trafficking.Curr Opin Cell Biol. 1994 Aug;6(4):522-6. doi: 10.1016/0955-0674(94)90071-x. Curr Opin Cell Biol. 1994. PMID: 7986528 Review.
-
[Protein isoprenylation].Postepy Biochem. 2004;50(4):316-29. Postepy Biochem. 2004. PMID: 15957527 Review. Polish. No abstract available.
Cited by
-
iNOS is necessary for GBP-mediated T. gondii clearance in murine macrophages via vacuole nitration and intravacuolar network collapse.Nat Commun. 2024 Mar 27;15(1):2698. doi: 10.1038/s41467-024-46790-y. Nat Commun. 2024. PMID: 38538595 Free PMC article.
-
Human guanylate binding proteins potentiate the anti-chlamydia effects of interferon-gamma.PLoS One. 2009 Aug 4;4(8):e6499. doi: 10.1371/journal.pone.0006499. PLoS One. 2009. PMID: 19652711 Free PMC article.
-
Detection of Cytosolic Shigella flexneri via a C-Terminal Triple-Arginine Motif of GBP1 Inhibits Actin-Based Motility.mBio. 2017 Dec 12;8(6):e01979-17. doi: 10.1128/mBio.01979-17. mBio. 2017. PMID: 29233899 Free PMC article.
-
Toxoplasma-proximal and distal control by GBPs in human macrophages.Pathog Dis. 2022 Jan 7;79(9):ftab058. doi: 10.1093/femspd/ftab058. Pathog Dis. 2022. PMID: 34931666 Free PMC article.
-
The GTPase activity of murine guanylate-binding protein 2 (mGBP2) controls the intracellular localization and recruitment to the parasitophorous vacuole of Toxoplasma gondii.J Biol Chem. 2012 Aug 10;287(33):27452-66. doi: 10.1074/jbc.M112.379636. Epub 2012 Jun 22. J Biol Chem. 2012. PMID: 22730319 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

