The NF1 tumor suppressor critically regulates TSC2 and mTOR
- PMID: 15937108
- PMCID: PMC1142482
- DOI: 10.1073/pnas.0503224102
The NF1 tumor suppressor critically regulates TSC2 and mTOR
Erratum in
- Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):16119
Abstract
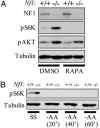
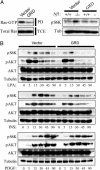
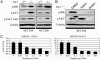
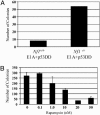
Loss-of-function mutations in the NF1 tumor suppressor gene underlie the familial cancer syndrome neurofibromatosis type I (NF1). The NF1-encoded protein, neurofibromin, functions as a Ras-GTPase activating protein (RasGAP). Accordingly, deregulation of Ras is thought to contribute to NF1 development. However, the critical effector pathways involved in disease pathogenesis are still unknown. We show here that the mTOR pathway is tightly regulated by neurofibromin. mTOR is constitutively activated in both NF1-deficient primary cells and human tumors in the absence of growth factors. This aberrant activation depends on Ras and PI3 kinase, and is mediated by the phosphorylation and inactivation of the TSC2-encoded protein tuberin by AKT. Importantly, tumor cell lines derived from NF1 patients, and a genetically engineered cell system that requires Nf1-deficiency for transformation, are highly sensitive to the mTOR inhibitor rapamycin. Furthermore, while we show that the activation of endogenous Ras leads to constitutive mTOR signaling in this disease state, we also demonstrate that in normal cells Ras is differentially required for mTOR signaling in response to various growth factors. Thus, these findings identify the NF1 tumor suppressor as an indispensable regulator of TSC2 and mTOR. Furthermore, our results also demonstrate that Ras plays a critical role in the activation of mTOR in both normal and tumorigenic settings. Finally, these data suggest that rapamycin, or its derivatives, may represent a viable therapy for NF1.
Figures

Similar articles
-
Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors.Cancer Res. 2005 Apr 1;65(7):2755-60. doi: 10.1158/0008-5472.CAN-04-4058. Cancer Res. 2005. PMID: 15805275
-
Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb.Curr Biol. 2003 Aug 5;13(15):1259-68. doi: 10.1016/s0960-9822(03)00506-2. Curr Biol. 2003. PMID: 12906785
-
Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase.Proc Natl Acad Sci U S A. 2004 Sep 14;101(37):13489-94. doi: 10.1073/pnas.0405659101. Epub 2004 Sep 1. Proc Natl Acad Sci U S A. 2004. PMID: 15342917 Free PMC article.
-
Rhebbing up mTOR: new insights on TSC1 and TSC2, and the pathogenesis of tuberous sclerosis.Cancer Biol Ther. 2003 Sep-Oct;2(5):471-6. doi: 10.4161/cbt.2.5.446. Cancer Biol Ther. 2003. PMID: 14614311 Review.
-
Molecular therapies for tuberous sclerosis and neurofibromatosis.Curr Neurol Neurosci Rep. 2012 Jun;12(3):294-301. doi: 10.1007/s11910-012-0269-4. Curr Neurol Neurosci Rep. 2012. PMID: 22544507 Review.
Cited by
-
Report from the 13th annual Western canadian gastrointestinal cancer consensus conference; calgary, alberta; september 8-10, 2011.Curr Oncol. 2012 Dec;19(6):e468-77. doi: 10.3747/co.19.1167. Curr Oncol. 2012. PMID: 23300370 Free PMC article.
-
Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth.Neuro Oncol. 2015 Jun;17(6):843-53. doi: 10.1093/neuonc/nou329. Epub 2014 Dec 21. Neuro Oncol. 2015. PMID: 25534823 Free PMC article.
-
Targeting the PI3K/mTOR axis, alone and in combination with autophagy blockade, for the treatment of malignant peripheral nerve sheath tumors.Mol Cancer Ther. 2012 Aug;11(8):1758-69. doi: 10.1158/1535-7163.MCT-12-0015. Epub 2012 Jul 30. Mol Cancer Ther. 2012. PMID: 22848094 Free PMC article.
-
Ras Signaling in Breast Cancer.Adv Exp Med Biol. 2021;1187:81-101. doi: 10.1007/978-981-32-9620-6_4. Adv Exp Med Biol. 2021. PMID: 33983574
-
Understanding the Biological Activities of Vitamin D in Type 1 Neurofibromatosis: New Insights into Disease Pathogenesis and Therapeutic Design.Cancers (Basel). 2020 Oct 13;12(10):2965. doi: 10.3390/cancers12102965. Cancers (Basel). 2020. PMID: 33066259 Free PMC article. Review.
References
-
- Riccardi, V. M. (1992) Neurofibromatosis: Phenotype, Natural History, and Pathogenesis (Johns Hopkins Univ. Press, Baltimore).
-
- Bader, J. L. (1986) Ann. N.Y. Acad. Sci. 486, 57-65. - PubMed
-
- Basu, T. N., Gutmann, D. H., Fletcher, J. A., Glover, T. W., Collins, F. S. & Downward, J. (1992) Nature 356, 713-715. - PubMed
-
- DeClue, J. E., Papageorge, A. G., Fletcher, J. A., Diehl, S. R., Ratner, N., Vass, W. C. & Lowy, D. R. (1992) Cell 69, 265-273. - PubMed
-
- Kim, H. A., Rosenbaum, T., Marchionni, M. A., Ratner, N. & DeClue, J. E. (1995) Oncogene 11, 325-335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

