Imaging individual retroviral fusion events: from hemifusion to pore formation and growth
- PMID: 15937118
- PMCID: PMC1150829
- DOI: 10.1073/pnas.0501864102
Imaging individual retroviral fusion events: from hemifusion to pore formation and growth
Abstract
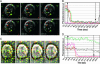
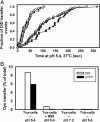
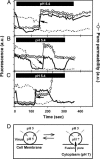
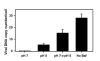
Viral fusion proteins catalyze merger of viral and cell membranes through a series of steps that have not yet been well defined. To elucidate the mechanism of virus entry, we have imaged fusion between single virions bearing avian sarcoma and leukosis virus (ASLV) envelope glycoprotein (Env) and the cell membrane. Viral particles were labeled with a lipophilic dye and with palmitylated enhanced YFP that was incorporated into the inner leaflet of the viral membrane. When individual virions were bound to target cells expressing cognate receptors, they transferred their lipids and contents only when exposed to low, but not neutral, pH. These data are consistent with the proposed two-step mechanism of ASLV entry that involves receptor-priming followed by low pH activation. Most importantly, lipid mixing commonly occurred before formation of a small fusion pore that was quickly and sensitively detected by pH-dependent changes in palmitylated enhanced YFP fluorescence. Nascent fusion pores were metastable and irreversibly closed, remained small, or fully enlarged, permitting nucleocapsid delivery into the cytosol. These findings strongly imply that hemifusion and a small pore are the key intermediates of ASLV fusion. When added before low pH treatment, a peptide designed to prevent Env from folding into a final helical-bundle conformation abolished virus-cell fusion and infection. Therefore, we conclude that, after receptor-activation, Env undergoes low pH-dependent refolding into a six-helix bundle and, in doing so, sequentially catalyzes hemifusion, fusion pore opening, and enlargement.
Figures

Similar articles
-
Low pH is required for avian sarcoma and leukosis virus Env-induced hemifusion and fusion pore formation but not for pore growth.J Virol. 2004 Apr;78(7):3753-62. doi: 10.1128/jvi.78.7.3753-3762.2004. J Virol. 2004. PMID: 15016895 Free PMC article.
-
A study of low pH-induced refolding of Env of avian sarcoma and leukosis virus into a six-helix bundle.Biophys J. 2004 Nov;87(5):3291-8. doi: 10.1529/biophysj.104.047696. Epub 2004 Aug 31. Biophys J. 2004. PMID: 15339808 Free PMC article.
-
The avian retrovirus avian sarcoma/leukosis virus subtype A reaches the lipid mixing stage of fusion at neutral pH.J Virol. 2003 Mar;77(5):3058-66. doi: 10.1128/jvi.77.5.3058-3066.2003. J Virol. 2003. PMID: 12584331 Free PMC article.
-
Alpharetrovirus envelope-receptor interactions.Curr Top Microbiol Immunol. 2003;281:107-36. doi: 10.1007/978-3-642-19012-4_3. Curr Top Microbiol Immunol. 2003. PMID: 12932076 Review.
-
Cell entry of enveloped viruses.Adv Genet. 2011;73:121-83. doi: 10.1016/B978-0-12-380860-8.00004-5. Adv Genet. 2011. PMID: 21310296 Free PMC article. Review.
Cited by
-
Targeting Human Proteins for Antiviral Drug Discovery and Repurposing Efforts: A Focus on Protein Kinases.Viruses. 2023 Feb 19;15(2):568. doi: 10.3390/v15020568. Viruses. 2023. PMID: 36851782 Free PMC article. Review.
-
The hr1 and fusion peptide regions of the subgroup B avian sarcoma and leukosis virus envelope glycoprotein influence low pH-dependent membrane fusion.PLoS One. 2007 Jan 24;2(1):e171. doi: 10.1371/journal.pone.0000171. PLoS One. 2007. PMID: 17245447 Free PMC article.
-
Studying Retroviral Life Cycles Using Visible Viruses and Live Cell Imaging.Annu Rev Virol. 2024 Sep;11(1):125-146. doi: 10.1146/annurev-virology-100422-012608. Epub 2024 Aug 30. Annu Rev Virol. 2024. PMID: 38876144 Free PMC article. Review.
-
Dynamics and restriction of murine leukemia virus cores in mitotic and interphase cells.Retrovirology. 2015 Nov 14;12:95. doi: 10.1186/s12977-015-0220-2. Retrovirology. 2015. PMID: 26577111 Free PMC article.
-
Single-cell imaging of HIV-1 provirus (SCIP).Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5636-41. doi: 10.1073/pnas.1216254110. Epub 2013 Mar 19. Proc Natl Acad Sci U S A. 2013. PMID: 23513220 Free PMC article.
References
-
- Chernomordik, L. V. & Kozlov, M. M. (2003) Annu. Rev. Biochem. 72, 175-207. - PubMed
-
- Jahn, R., Lang, T. & Sudhof, T. C. (2003) Cell 112, 519-533. - PubMed
-
- Takahashi, N., Kishimoto, T., Nemoto, T., Kadowaki, T. & Kasai, H. (2002) Science 297, 1349-1352. - PubMed
-
- Xu, Y., Zhang, F., Su, Z., McNew, J. A. & Shin, Y. K. (2005) Nat. Struct. Mol. Biol. 12, 417-422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

