Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle
- PMID: 15937120
- PMCID: PMC1142481
- DOI: 10.1073/pnas.0500530102
Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle
Abstract
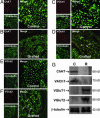
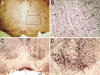
Acetylcholine is the main neurotransmitter at the mammalian neuromuscular junction (NMJ) where nicotinic acetylcholine receptors mediate the signaling between nerve terminals and muscle fibers. We show that under glutamatergic transmission, rat NMJ switches from cholinergic type synapse to glutamatergic synapse. Connecting skeletal muscle to the lateral white matter of the spinal cord by grafting the distal stump of the transected motor nerve produced functional muscle reinnervation. The restored neuromuscular activity became resistant to common curare blockers but sensitive to the glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist. Analysis of the regenerated nerve disclosed new glutamatergic axons and the disappearance of cholinergic fibers. Many axons belonged to the supraspinal neurons located in the red nucleus and the brainstem nuclei. Finally, the innervated muscle displayed high expression and clustering of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunits glutamate receptors 1 and 2. Our data suggest that supraspinal neurons can target skeletal muscle, which retains the plasticity to generate functional glutamatergic NMJ.
Figures


References
-
- Schwab, M. E. & Bartholdi, D. (1996) Physiol. Rev. 76, 319-370. - PubMed
-
- Fawcett, J. W. & Asher, R. A. (1999) Brain Res. Bull. 49, 377-391. - PubMed
-
- Silver, J. & Miller, J. H. (2004) Nat. Rev. Neurosci. 5, 146-156. - PubMed
-
- Richardson, P. M., McGuinness, U. M. & Aguayo, A. J. (1980) Nature 284, 264-265. - PubMed
-
- David, S. & Aguayo, A. J. (1981) Science 214, 931-933. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

