Biochemical characterization of a prokaryotic phenylalanine ammonia lyase
- PMID: 15937191
- PMCID: PMC1151709
- DOI: 10.1128/JB.187.12.4286-4289.2005
Biochemical characterization of a prokaryotic phenylalanine ammonia lyase
Erratum in
- J Bacteriol. 2006 Jul;188(14):5331
Abstract
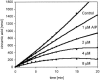
The committed biosynthetic reaction to benzoyl-coenzyme A in the marine bacterium "Streptomyces maritimus" is carried out by the novel prokaryotic phenylalanine ammonia lyase (PAL) EncP, which converts the primary amino acid L-phenylalanine to trans-cinnamic acid. Recombinant EncP is specific for L-phenylalanine and shares many biochemical features with eukaryotic PALs, which are substantially larger proteins by approximately 200 amino acid residues.
Figures



References
-
- Appert, C., J. Zon, and N. Amrhein. 2003. Kinetic analysis of the inhibition of phenylalanine ammonia-lyase by 2-aminoindan-2-phosphonic acid and other phenylalanine analogues. Phytochemistry 62:415-422. - PubMed
-
- Bezanson, G. S., D. Desaty, A. V. Emes, and L. C. Vining. 1970. Biosynthesis of cinnamamide and detection of phenylalanine ammonia-lyase in Streptomyces verticillatus. Can. J. Microbiol. 16:147-151. - PubMed
-
- Calabrese, J. C., D. B. Jordan, A. Boodhoo, S. Sariaslani, and T. Vannelli. 2004. Crystal structure of phenylalanine ammonia lyase: multiple helix dipoles implicated in catalysis. Biochemistry 43:11403-11416. - PubMed
-
- Christenson, S. D., W. Liu, M. D. Toney, and B. Shen. 2003. A novel 4-methylideneimidazole-5-one-containing tyrosine aminomutase in enediyne antitumor antibiotic C-1027 biosynthesis. J. Am. Chem. Soc. 125:6062-6063. - PubMed
-
- Christenson, S. D., W. Wu, M. A. Spies, B. Shen, and M. D. Toney. 2003. Kinetic analysis of the 4-methylideneimidazole-5-one-containing tyrosine aminomutase in enediyne antitumor antibiotic C-1027 biosynthesis. Biochemistry 42:12708-12718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

