Analysis of a noncanonical poly(A) site reveals a tripartite mechanism for vertebrate poly(A) site recognition
- PMID: 15937220
- PMCID: PMC1142555
- DOI: 10.1101/gad.1298605
Analysis of a noncanonical poly(A) site reveals a tripartite mechanism for vertebrate poly(A) site recognition
Abstract
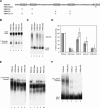
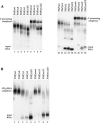
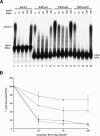
At least half of all human pre-mRNAs are subject to alternative 3' processing that may modulate both the coding capacity of the message and the array of post-transcriptional regulatory elements embedded within the 3' UTR. Vertebrate poly(A) site selection appears to rely primarily on the binding of CPSF to an A(A/U)UAAA hexamer upstream of the cleavage site and CstF to a downstream GU-rich element. At least one-quarter of all human poly(A) sites, however, lack the A(A/U)UAAA motif. We report that sequence-specific RNA binding of the human 3' processing factor CFI(m) can function as a primary determinant of poly(A) site recognition in the absence of the A(A/U)UAAA motif. CFI(m) is sufficient to direct sequence-specific, A(A/U)UAAA-independent poly(A) addition in vitro through the recruitment of the CPSF subunit hFip1 and poly(A) polymerase to the RNA substrate. ChIP analysis indicates that CFI(m) is recruited to the transcription unit, along with CPSF and CstF, during the initial stages of transcription, supporting a direct role for CFI(m) in poly(A) site recognition. The recognition of three distinct sequence elements by CFI(m), CPSF, and CstF suggests that vertebrate poly(A) site definition is mechanistically more similar to that of yeast and plants than anticipated.
Figures




References
-
- Ahn S.H., Kim, M., and Buratowski, S. 2004. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol. Cell 13: 67-76. - PubMed
-
- Aissouni Y., Perez, C., Calmels, B., and Benech, P.D. 2002. The cleavage/polyadenylation activity triggered by a U-rich motif sequence is differently required depending on the poly(A) site location at either the first or last 3′-terminal exon of the 2 ′-5′ oligo(A) synthetase gene. J. Biol. Chem. 277: 35808-35814. - PubMed
-
- Barabino S.M.L. and Keller, W. 1999. Last but not least: Regulated poly(A) tail formation. Cell 99: 9-11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
