Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation
- PMID: 15937223
- PMCID: PMC1142560
- DOI: 10.1101/gad.329705
Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation
Abstract
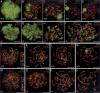
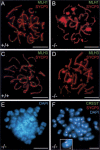
In meiotic prophase, synaptonemal complexes (SCs) closely appose homologous chromosomes (homologs) along their length. SCs are assembled from two axial elements (AEs), one along each homolog, which are connected by numerous transverse filaments (TFs). We disrupted the mouse gene encoding TF protein Sycp1 to analyze the role of TFs in meiotic chromosome behavior and recombination. Sycp1(-/-) mice are infertile, but otherwise healthy. Sycp1(-/-) spermatocytes form normal AEs, which align homologously, but do not synapse. Most Sycp1(-/-) spermatocytes arrest in pachynema, whereas a small proportion reaches diplonema, or, exceptionally, metaphase I. In leptotene Sycp1(-/-) spermatocytes, gammaH2AX (indicative of DNA damage, including double-strand breaks) appears normal. In pachynema, Sycp1(-/-) spermatocytes display a number of discrete gammaH2AX domains along each chromosome, whereas gammaH2AX disappears from autosomes in wild-type spermatocytes. RAD51/DMC1, RPA, and MSH4 foci (which mark early and intermediate steps in pairing/recombination) appear in similar numbers as in wild type, but do not all disappear, and MLH1 and MLH3 foci (which mark late steps in crossing over) are not formed. Crossovers were rare in metaphase I of Sycp1(-/-) mice. We propose that SYCP1 has a coordinating role, and ensures formation of crossovers. Unexpectedly, Sycp1(-/-) spermatocytes did not form XY bodies.
Figures




References
-
- Alpi A., Pasierbek, P., Gartner, A., and Loidl, J. 2003. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112: 6-16. - PubMed
-
- Ashley T. 2004. The mouse “tool box” for meiotic studies. Cytogenet. Genome Res. 105: 166-171. - PubMed
-
- Ashley T. and Plug, A.W. 1998. Caught in the act: Deducing meiotic function from protein immunolocalization. Curr. Top. Dev. Biol. 37: 201-239. - PubMed
-
- Baarends W., Wassenaar, E., Hoogerbrugge, J.W., van Capellen, G., Roest, H.P., Vreeburg, J., Ooms, M., Hoeijmakers, J.H.J., and Grootegoed, J.A. 2003. Loss of HR6B ubiquitin-conjugating activity results in damaged synaptonemal complex structure and increased crossing-over frequency during the male meiotic prophase. Mol. Cell. Biol. 23: 1151-1162. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
