Cellular responses to nicotinic receptor activation are decreased after prolonged exposure to galantamine in human neuroblastoma cells
- PMID: 15937519
- PMCID: PMC1576228
- DOI: 10.1038/sj.bjp.0706278
Cellular responses to nicotinic receptor activation are decreased after prolonged exposure to galantamine in human neuroblastoma cells
Abstract
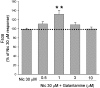
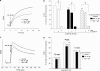
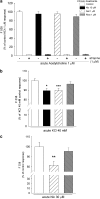
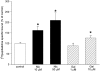
In this study, we have examined cellular responses of neuroblastoma SH-SY5Y cells after chronic treatment with galantamine, a drug used to treat Alzheimer's disease that has a dual mechanism of action: inhibition of acetylcholinesterase and allosteric potentiation of nicotinic acetylcholine receptors (nAChR). Acute experiments confirmed that maximum potentiation of nicotinic responses occurs at 1 microM galantamine; hence this concentration was chosen for chronic treatment. Exposure to 1 microM galantamine for 4 days decreased Ca(2+) responses (by 19.8+/-3.6%) or [(3)H]noradrenaline ([(3)H]NA) release (by 23.9+/-3.3%) elicited by acute application of nicotine. KCl-evoked increases in intracellular Ca(2+) were also inhibited by 10.0+/-1.9% after 4 days' treatment with galantamine. These diminished responses are consistent with the downregulation of downstream cellular processes. Ca(2+) responses evoked by activation of muscarinic acetylcholine receptors were unaffected by chronic galantamine treatment. Exposure to the more potent acetylcholinesterase inhibitor rivastigmine (1 microM) for 4 days failed to alter nicotine-, KCl-, or muscarinic receptor-evoked increases in intracellular Ca(2+). These observations support the hypothesis that chronic galantamine exerts its effects through interaction with nAChR in this cell line. Exposure to 10 microM nicotine for 4 days produced decreases in acute nicotine- (18.0+/-3.5%) and KCl-evoked Ca(2+) responses (10.6+/-2.5%) and nicotine-evoked [(3)H]NA release (26.0+/-3.3%) that are comparable to the effects of a corresponding exposure to galantamine. Treatment with 1 microM galantamine did not alter numbers of [(3)H]epibatidine-binding sites in SH-SY5Y cells, in contrast to 62% upregulation of these sites in response to 10 microM nicotine. Thus, chronic galantamine acts at nAChR to decrease subsequent functional responses to acute stimulation with nicotine or KCl. This effect appears to be independent of the upregulation of nAChR-binding sites.
Figures


References
-
- AGIS-TORRES A., BALL S.G., VAUGHAN P.F. Chronic treatment with nicotine or potassium attenuates depolarisation-evoked noradrenaline release from the human neuroblastoma SH-SY5Y. Neurosci. Lett. 2002;331:167–170. - PubMed
-
- ARIAS E., ALES E., GABILAN N.H., CANO-ABAD M.F., VILLARROYA M., GARCIA A.G., LOPEZ M.G. Galantamine prevents apoptosis induced by beta-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology. 2004;46:103–114. - PubMed
-
- BARNES C.A., MELTZER J., HOUSTON F., ORR G., MCGANN K., WENK G.L. Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience. 2000;99:17–23. - PubMed
-
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

