Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis
- PMID: 15937546
- PMCID: PMC1142114
- DOI: 10.1172/JCI24399
Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis
Abstract
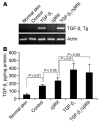
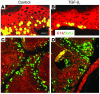
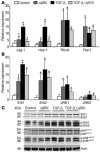
In the present study, we demonstrated that human skin cancers frequently overexpress TGF-beta1 but exhibit decreased expression of the TGF-beta type II receptor (TGF-(beta)RII). To understand how this combination affects cancer prognosis, we generated a transgenic mouse model that allowed inducible expression of TGF-beta(1) in keratinocytes expressing a dominant negative TGF-(beta)RII (Delta(beta)RII) in the epidermis. Without Delta(beta)RII expression, TGF-beta1 transgene induction in late-stage, chemically induced papillomas failed to inhibit tumor growth but increased metastasis and epithelial-to-mesenchymal transition (EMT), i.e., formation of spindle cell carcinomas. Interestingly, Delta(beta)RII expression abrogated TGF-beta1-mediated EMT and was accompanied by restoration of membrane-associated E-cadherin/catenin complex in TGF-beta1/Delta(beta)RII compound tumors. Furthermore, expression of molecules thought to mediate TGF-beta1-induced EMT was attenuated in TGF-beta1/Delta(beta)RII-transgenic tumors. However, TGF-beta1/Delta(beta)RII-transgenic tumors progressed to metastasis without losing expression of the membrane-associated E-cadherin/catenin complex and at a rate higher than those observed in nontransgenic, TGF-beta1-transgenic, or Delta(beta)RII-transgenic mice. Abrogation of Smad activation by Delta(beta)RII correlated with the blockade of EMT. However, Delta(beta)RII did not alter TGF-beta1-mediated expression of RhoA/Rac and MAPK, which contributed to increased metastasis. Our study provides evidence that TGF-beta1 induces EMT and invasion via distinct mechanisms. TGF-beta1-mediated EMT requires functional TGF-(beta)RII, whereas TGF-beta1-mediated tumor invasion cooperates with reduced TGF-(beta)RII signaling in tumor epithelia.
Figures






Similar articles
-
Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis.Cancer Res. 2001 Oct 15;61(20):7435-43. Cancer Res. 2001. PMID: 11606377
-
Blocking transforming growth factor beta signaling in transgenic epidermis accelerates chemical carcinogenesis: a mechanism associated with increased angiogenesis.Cancer Res. 1999 Jun 15;59(12):2861-8. Cancer Res. 1999. PMID: 10383147
-
Transforming growth factor-β and epithelial-mesenchymal transition are associated with pulmonary metastasis in adenoid cystic carcinoma.Oral Oncol. 2013 Nov;49(11):1051-8. doi: 10.1016/j.oraloncology.2013.07.012. Epub 2013 Aug 17. Oral Oncol. 2013. PMID: 23962790
-
Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer.Biochim Biophys Acta. 2007 Jan;1775(1):21-62. doi: 10.1016/j.bbcan.2006.06.004. Epub 2006 Jul 8. Biochim Biophys Acta. 2007. PMID: 16904831 Review.
-
Current view of the role of transforming growth factor beta 1 in skin carcinogenesis.J Investig Dermatol Symp Proc. 2005 Nov;10(2):110-7. doi: 10.1111/j.1087-0024.2005.200403.x. J Investig Dermatol Symp Proc. 2005. PMID: 16363062 Review.
Cited by
-
Crossing paths in Human Renal Cell Carcinoma (hRCC).Int J Mol Sci. 2012 Oct 5;13(10):12710-33. doi: 10.3390/ijms131012710. Int J Mol Sci. 2012. PMID: 23202921 Free PMC article. Review.
-
DGKZ promotes TGFβ signaling pathway and metastasis in triple-negative breast cancer by suppressing lipid raft-dependent endocytosis of TGFβR2.Cell Death Dis. 2022 Feb 3;13(2):105. doi: 10.1038/s41419-022-04537-x. Cell Death Dis. 2022. PMID: 35115500 Free PMC article.
-
Transforming growth factor beta controls the directional migration of hepatocyte cohorts by modulating their adhesion to fibronectin.Mol Biol Cell. 2008 Mar;19(3):945-56. doi: 10.1091/mbc.e07-09-0967. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094041 Free PMC article.
-
TGF-beta1-Induced Expression of the Poor Prognosis SERPINE1/PAI-1 Gene Requires EGFR Signaling: A New Target for Anti-EGFR Therapy.J Oncol. 2009;2009:342391. doi: 10.1155/2009/342391. Epub 2009 Apr 9. J Oncol. 2009. PMID: 19365582 Free PMC article.
-
CENP-R acts bilaterally as a tumor suppressor and as an oncogene in the two-stage skin carcinogenesis model.Cancer Sci. 2017 Nov;108(11):2142-2148. doi: 10.1111/cas.13348. Epub 2017 Aug 30. Cancer Sci. 2017. PMID: 28795467 Free PMC article.
References
-
- Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit. Rev. Oncog. 1999;10:303–360. - PubMed
-
- Reiss M. TGF-beta and cancer. Microbes Infect. 1999;1:1327–1347. - PubMed
-
- Lu SL, et al. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 2004;64:4405–4410. - PubMed
-
- Wang XJ. Role of TGFbeta signaling in skin carcinogenesis. Microsc. Res. Tech. 2001;52:420–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

