Metal-enhanced fluorescence using anisotropic silver nanostructures: critical progress to date
- PMID: 15937664
- PMCID: PMC6844248
- DOI: 10.1007/s00216-005-3195-3
Metal-enhanced fluorescence using anisotropic silver nanostructures: critical progress to date
Abstract
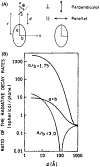
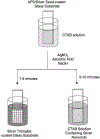
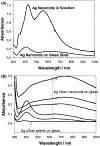
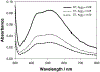
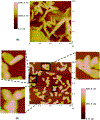
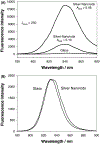
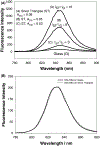
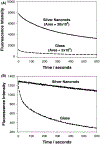
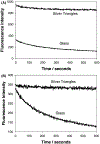
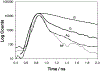
In this critical and timely review, the effects of anisotropic silver nanostructures on the emission intensity and photostability of a key fluorophore that is frequently used in many biological assays is examined. The silver nanostructures consist of triangular, rod-like, and fractal-like nanoparticles of silver deposited on conventional glass substrates. The close proximity to silver nanostructures results in greater intensity and photostability of the fluorophore than for fluorophores solely deposited on glass substrates. These new anisotropic silver nanostructure-coated surfaces show much more favorable effects than silver island films or silver colloid-coated substrates. Subsequently, the use of metal-enhanced fluorescence (MEF) for biosensing applications is discussed.
Figures


Similar articles
-
Fast and slow deposition of silver nanorods on planar surfaces: application to metal-enhanced fluorescence.J Phys Chem B. 2005 Mar 3;109(8):3157-62. doi: 10.1021/jp045186t. J Phys Chem B. 2005. PMID: 16851335 Free PMC article.
-
Angular-dependent metal-enhanced fluorescence from silver colloid-deposited films: opportunity for angular-ratiometric surface assays.Analyst. 2007 Nov;132(11):1112-21. doi: 10.1039/b709170b. Epub 2007 Aug 23. Analyst. 2007. PMID: 17955145
-
Metal-enhanced fluorescence from silver-SiO2-silver nanoburger structures.Langmuir. 2010 Jul 20;26(14):12371-6. doi: 10.1021/la101801n. Langmuir. 2010. PMID: 20486652
-
Advances in three dimensional metal enhanced fluorescence based biosensors using metal nanomaterial and nano-patterned surfaces.Biotechnol J. 2024 Jan;19(1):e2300519. doi: 10.1002/biot.202300519. Epub 2023 Dec 5. Biotechnol J. 2024. PMID: 37997672 Review.
-
Advances in surface-enhanced fluorescence.J Fluoresc. 2004 Jul;14(4):425-41. doi: 10.1023/b:jofl.0000031824.48401.5c. J Fluoresc. 2004. PMID: 15617385 Free PMC article. Review.
Cited by
-
Fluorescence Quenching of CdTe Nanocrystals by Bound Gold Nanoparticles in Aqueous Solution.Plasmonics. 2008 Mar 1;3(1):3-11. doi: 10.1007/s11468-007-9047-6. Plasmonics. 2008. PMID: 19890452 Free PMC article.
-
Shining Light on Chitosan: A Review on the Usage of Chitosan for Photonics and Nanomaterials Research.Int J Mol Sci. 2018 Jun 17;19(6):1795. doi: 10.3390/ijms19061795. Int J Mol Sci. 2018. PMID: 29914214 Free PMC article. Review.
-
Insight into factors affecting the presence, degree, and temporal stability of fluorescence intensification on ZnO nanorod ends.Nanoscale. 2015 Jan 28;7(4):1424-36. doi: 10.1039/c4nr06066k. Nanoscale. 2015. PMID: 25504319 Free PMC article.
-
Metal-enhanced chemiluminescence: advanced chemiluminescence concepts for the 21st century.Chem Soc Rev. 2009 Sep;38(9):2556-64. doi: 10.1039/b807498b. Epub 2009 Jun 10. Chem Soc Rev. 2009. PMID: 19690736 Free PMC article. Review.
-
Fluorescent core-shell Ag@SiO2 nanocomposites for metal-enhanced fluorescence and single nanoparticle sensing platforms.J Am Chem Soc. 2007 Feb 14;129(6):1524-5. doi: 10.1021/ja0680820. J Am Chem Soc. 2007. PMID: 17283994 Free PMC article. No abstract available.
References
-
- Gersten J, Nitzan A (1981) J Chem Phys 75:1139–1152
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

