Neurogenesis and ontogeny of specific cell phenotypes within the hamster suprachiasmatic nucleus
- PMID: 15939080
- PMCID: PMC3275417
- DOI: 10.1016/j.devbrainres.2005.02.017
Neurogenesis and ontogeny of specific cell phenotypes within the hamster suprachiasmatic nucleus
Abstract
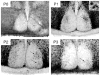
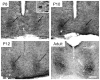
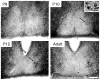
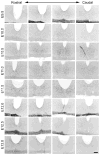
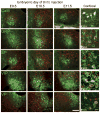
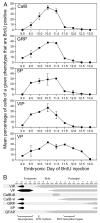
The hamster suprachiasmatic nucleus (SCN) is anatomically and functionally heterogeneous. A group of cells in the SCN shell, delineated by vasopressin-ergic neurons, are rhythmic with respect to Period gene expression and electrical activity but do not receive direct retinal input. In contrast, some cells in the SCN core, marked by neurons containing calbindin-D28k, gastrin-releasing peptide (GRP), substance P (SP), and vasoactive intestinal polypeptide (VIP), are not rhythmic with respect to Period gene expression and electrical activity but do receive direct retinal input. Examination of the timing of neurogenesis using bromodeoxyuridine indicates that SCN cells are born between embryonic day 9.5 and 12.5. Calbindin, GRP, substance P, and VIP cells are born only during early SCN neurogenesis, between embryonic days 9.5-11.0. Vasopressin cells are born over the whole period of SCN neurogenesis, appearing as late as embryonic day 12.5. Examination of the ontogeny of peptide expression in these cell types reveals transient expression of calbindin in a cluster of dorsolateral SCN cells on postnatal days 1-2. The adult pattern of calbindin expression is detected in a different ventrolateral cell cluster starting on postnatal day 2. GRP and SP expression appear on postnatal day 8 and 10, respectively, after the retinohypothalamic tract has innervated the SCN. In summary, the present study describes the ontogeny-specific peptidergic phenotypes in the SCN and compares these developmental patterns to previously identified patterns in the appearance of circadian functions. These comparisons suggest the possibility that these coincident appearances may be causally related, with the direction of causation to be determined.
Figures
References
-
- Abizaid A, Mezei G, Sotonyi P, Horvath TL. Sex differences in adult suprachiasmatic nucleus neurons emerging late prenatally in rats. Eur J Neurosci. 2004;19:2488–2496. - PubMed
-
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol. 2002;61:26–34. - PubMed
-
- Altman J, Bayer SA. Development of the diencephalon in the rat: I. Autoradiographic study of the time of origin and settling patterns of neurons of the hypothalamus. J Comp Neurol. 1978;182:945–971. - PubMed
-
- Antle MC, Silver R. Orchestrating time: Arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

