Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins
- PMID: 15939738
- PMCID: PMC1747414
- DOI: 10.1074/jbc.M503957200
Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins
Abstract
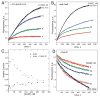
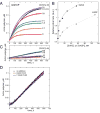
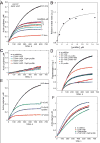
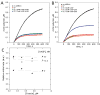
Ena/VASP proteins influence the organization of actin filament networks within lamellipodia and filopodia of migrating cells and in actin comet tails. The molecular mechanisms by which Ena/VASP proteins control actin dynamics are unknown. We investigated how Ena/VASP proteins regulate actin polymerization at actin filament barbed ends in vitro in the presence and absence of barbed end capping proteins. Recombinant His-tagged VASP increased the rate of actin polymerization in the presence of the barbed end cappers, heterodimeric capping protein (CP), CapG, and gelsolin-actin complex. Profilin enhanced the ability of VASP to protect barbed ends from capping by CP, and this required interactions of profilin with G-actin and VASP. The VASP EVH2 domain was sufficient to protect barbed ends from capping, and the F-actin and G-actin binding motifs within EVH2 were required. Phosphorylation by protein kinase A at sites within the VASP EVH2 domain regulated anti-capping and F-actin bundling by VASP. We propose that Ena/VASP proteins associate at or near actin filament barbed ends, promote actin assembly, and restrict the access of barbed end capping proteins.
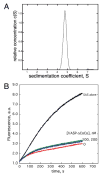
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

