Protective antigen and toxin neutralization antibody patterns in anthrax vaccinees undergoing serial plasmapheresis
- PMID: 15939745
- PMCID: PMC1151968
- DOI: 10.1128/CDLI.12.6.713-721.2005
Protective antigen and toxin neutralization antibody patterns in anthrax vaccinees undergoing serial plasmapheresis
Abstract
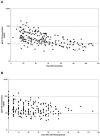
Recipients of licensed anthrax vaccine (AVA; Biothrax) could serve as a source of hyperimmune plasma and immunoglobulin for therapy and prophylaxis. We measured serum antibodies during serial weekly to biweekly plasmapheresis in 38 individuals previously vaccinated with 4 to 27 doses of AVA. Immunoglobulin G (IgG) to protective antigen (PA) and toxin neutralization assay (TNA) antibody levels were highly correlated (r = 0.86930 and P < 0.0001 for anti-PA concentration versus TNA concentration). Significant decreases in antibody titer and concentration were observed over time when compared for the number of days from the last AVA injection (P < 0.0001 for both anti-PA and TNA concentration) and for the number of days from the first plasmapheresis (P = 0.0007 for anti-PA concentration and P = 0.0025 for TNA concentration). The rate of the decrease in total IgG concentration (half-life [t(1/2)] = 198.90 days after first plasmapheresis) was significantly less than the decrease in anti-PA IgG (t(1/2) = 63.53 days) (P < 0.0001), indicating that the reduction in anti-PA IgG was more likely due to natural decay than plasmapheresis. The time since the last injection and the time after initial plasmapheresis are important elements in considering an optimal schedule for collecting anthrax hyperimmune plasma. Good correlation between IgG to PA and TNA antibodies suggests that the anti-PA enzyme-linked immunosorbent assay can be used as a high-throughput screen for functional immune reactivity in donor plasma units.
Figures
Similar articles
-
A three-dose intramuscular injection schedule of anthrax vaccine adsorbed generates sustained humoral and cellular immune responses to protective antigen and provides long-term protection against inhalation anthrax in rhesus macaques.Clin Vaccine Immunol. 2012 Nov;19(11):1730-45. doi: 10.1128/CVI.00324-12. Epub 2012 Aug 29. Clin Vaccine Immunol. 2012. PMID: 22933399 Free PMC article.
-
Anthrax Vaccine Precipitated Induces Edema Toxin-Neutralizing, Edema Factor-Specific Antibodies in Human Recipients.Clin Vaccine Immunol. 2017 Nov 6;24(11):e00165-17. doi: 10.1128/CVI.00165-17. Print 2017 Nov. Clin Vaccine Immunol. 2017. PMID: 28877928 Free PMC article.
-
Anthrax vaccine adsorbed: further evidence supporting continuing the vaccination series rather than restarting the series when doses are delayed.Vaccine. 2014 Sep 3;32(39):5131-9. doi: 10.1016/j.vaccine.2014.03.076. Epub 2014 May 14. Vaccine. 2014. PMID: 24837771
-
Effect of delayed anthrax vaccine dose on Bacillus anthracis protective antigen IgG response and lethal toxin neutralization activity.Vaccine. 2013 Oct 17;31(44):5009-14. doi: 10.1016/j.vaccine.2013.08.086. Epub 2013 Sep 8. Vaccine. 2013. PMID: 24026013
-
Neutralizing activity of vaccine-induced antibodies to two Bacillus anthracis toxin components, lethal factor and edema factor.Clin Vaccine Immunol. 2008 Jan;15(1):71-5. doi: 10.1128/CVI.00321-07. Epub 2007 Nov 21. Clin Vaccine Immunol. 2008. PMID: 18032590 Free PMC article.
Cited by
-
Characterization of the UK anthrax vaccine and human immunogenicity.Hum Vaccin Immunother. 2021 Mar 4;17(3):747-758. doi: 10.1080/21645515.2020.1799668. Epub 2020 Sep 8. Hum Vaccin Immunother. 2021. PMID: 32897798 Free PMC article.
-
CD1d-dependent B-cell help by NK-like T cells leads to enhanced and sustained production of Bacillus anthracis lethal toxin-neutralizing antibodies.Infect Immun. 2010 Apr;78(4):1610-7. doi: 10.1128/IAI.00002-10. Epub 2010 Feb 1. Infect Immun. 2010. PMID: 20123711 Free PMC article.
-
Recombinant Bacillus anthracis spore proteins enhance protection of mice primed with suboptimal amounts of protective antigen.Vaccine. 2008 Sep 8;26(38):4927-39. doi: 10.1016/j.vaccine.2008.07.015. Epub 2008 Jul 25. Vaccine. 2008. PMID: 18657585 Free PMC article.
-
A heterologous helper T-cell epitope enhances the immunogenicity of a multiple-antigenic-peptide vaccine targeting the cryptic loop-neutralizing determinant of Bacillus anthracis protective antigen.Infect Immun. 2009 Dec;77(12):5509-18. doi: 10.1128/IAI.00899-09. Epub 2009 Oct 5. Infect Immun. 2009. PMID: 19805525 Free PMC article.
-
Stochastic humoral immunity to Bacillus anthracis protective antigen: identification of anti-peptide IgG correlating with seroconversion to Lethal Toxin neutralization.Vaccine. 2013 Apr 3;31(14):1856-63. doi: 10.1016/j.vaccine.2013.01.040. Epub 2013 Feb 13. Vaccine. 2013. PMID: 23415781 Free PMC article.
References
-
- Arnon, S. S., R. Schechter, T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, J. Hauer, M. Layton, S. Lillibridge, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, D. L. Swerdlow, K. Tonat, and the Working Group on Civilian Biodefense. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285:1059-1070. - PubMed
-
- Brunell, P. A., A. A. Gershon, W. T. Hughes, H. D. Riley, Jr., and J. Smith. 1972. Prevention of varicella in high risk children: a collaborative study. Pediatrics 50:18-22. - PubMed
-
- Burgin, M., G. Hopkins, B. Moore, J. Nasser, A. Richardson, and R. Minchinton. 1992. Serum IgG and IgM levels in new and regular long-term plasmapheresis donors. Med. Lab. Sci. 49:265-270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

