A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells
- PMID: 15939762
- PMCID: PMC2171605
- DOI: 10.1083/jcb.200407123
A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells
Abstract
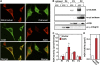
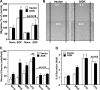
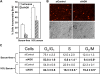
The bioactive phospholipids, lysophosphatidic acid (LPA) and phosphatidic acid (PA), regulate pivotal processes related to the pathogenesis of cancer. Here, we report characterization of a novel lipid kinase, designated acylglycerol kinase (AGK), that phosphorylates monoacylglycerol and diacylglycerol to form LPA and PA, respectively. Confocal microscopy and subcellular fractionation suggest that AGK is localized to the mitochondria. AGK expression was up-regulated in prostate cancers compared with normal prostate tissues from the same patient. Expression of AGK in PC-3 prostate cancer cells markedly increased formation and secretion of LPA. This increase resulted in concomitant transactivation of the EGF receptor and sustained activation of extracellular signal related kinase (ERK) 1/2, culminating in enhanced cell proliferation. AGK expression also increased migratory responses. Conversely, down-regulating expression of endogenous AGK inhibited EGF- but not LPA-induced ERK1/2 activation and progression through the S phase of the cell cycle. Hence, AGK can amplify EGF signaling pathways and may play an important role in the pathophysiology of prostate cancer.
Figures
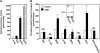




References
-
- Baker, D.L., D.M. Desiderio, D.D. Miller, B. Tolley, and G.J. Tigyi. 2001. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal. Biochem. 292:287–295. - PubMed
-
- Bookout, A.L., A.E. Finney, R. Guo, K. Peppel, W.J. Koch, and Y. Daaka. 2003. Targeting Gbetagamma signaling to inhibit prostate tumor formation and growth. J. Biol. Chem. 278:37569–37573. - PubMed
-
- Bunting, M., W. Tang, G.A. Zimmerman, T.M. McIntyre, and S.M. Prescott. 1996. Molecular cloning and characterization of a novel human diacylglycerol kinase ζ. J. Biol. Chem. 271:10230–10236. - PubMed
-
- Chakraborty, T.R., A. Vancura, V.S. Balija, and D. Haldar. 1999. Phosphatidic acid synthesis in mitochondria. Topography of formation and transmembrane migration. J. Biol. Chem. 274:29786–29790. - PubMed
-
- Fang, Y., M. Vilella-Bach, R. Bachmann, A. Flanigan, and J. Chen. 2001. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 294:1942–1945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

