The role of bioreductive activation of doxorubicin in cytotoxic activity against leukaemia HL60-sensitive cell line and its multidrug-resistant sublines
- PMID: 15942634
- PMCID: PMC2361480
- DOI: 10.1038/sj.bjc.6602639
The role of bioreductive activation of doxorubicin in cytotoxic activity against leukaemia HL60-sensitive cell line and its multidrug-resistant sublines
Abstract
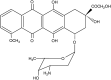
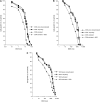
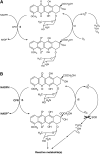
Clinical usefulness of doxorubicin (DOX) is limited by the occurrence of multidrug resistance (MDR) associated with the presence of membrane transporters (e.g. P-glycoprotein, MRP1) responsible for the active efflux of drugs out of resistant cells. Doxorubicin is a well-known bioreductive antitumour drug. Its ability to undergo a one-electron reduction by cellular oxidoreductases is related to the formation of an unstable semiquionone radical and followed by the production of reactive oxygen species. There is an increasing body of evidence that the activation of bioreductive drugs could result in the alkylation or crosslinking binding of DNA and lead to the significant increase in the cytotoxic activity against tumour cells. The aim of this study was to examine the role of reductive activation of DOX by the human liver NADPH cytochrome P450 reductase (CPR) in increasing its cytotoxic activity especially in regard to MDR tumour cells. It has been evidenced that, upon CPR catalysis, DOX underwent only the redox cycling (at low NADPH concentration) or a multistage chemical transformation (at high NADPH concentration). It was also found, using superoxide dismutase (SOD), that the first stage undergoing reductive activation according to the mechanism of the redox cycling had the key importance for the metabolic conversion of DOX. In the second part of this work, the ability of DOX to inhibit the growth of human promyelocytic-sensitive leukaemia HL60 cell line as well as its MDR sublines exhibiting two different phenotypes of MDR related to the overexpression of P-glycoprotein (HL60/VINC) or MRP1 (HL60/DOX) was studied in the presence of exogenously added CPR. Our assays showed that the presence of CPR catalysing only the redox cycling of DOX had no effect in increasing its cytotoxicity against sensitive and MDR tumour cells. In contrast, an important increase in cytotoxic activity of DOX after its reductive conversion by CPR was observed against HL60 as well as HL60/VINC and HL60/DOX cells.
Figures


Similar articles
-
The ability of selected pyridinium salts to increase the cytotoxic activity of vincristine but not doxorubicin towards sensitive and multidrug resistant promyelocytic leukaemia HL60 cells.J Pharm Pharmacol. 2008 May;60(5):647-53. doi: 10.1211/jpp.60.5.0011. J Pharm Pharmacol. 2008. PMID: 18416942
-
The role of bioreductive activation of antitumour anthracycline drugs in cytotoxic activity against sensitive and multidrug resistant leukaemia HL60 cells.Eur J Pharmacol. 2012 Jan 15;674(2-3):112-25. doi: 10.1016/j.ejphar.2011.10.047. Epub 2011 Nov 15. Eur J Pharmacol. 2012. PMID: 22115891
-
Role of structural factors of antitumour anthraquinone derivatives and analogues in the ability to undergo bioreductive activation by NADPH cytochrome P450 reductase: implications for increasing the activity against sensitive and multidrug-resistant leukaemia HL60 cells.Anticancer Drugs. 2012 Apr;23(4):393-405. doi: 10.1097/CAD.0b013e32834fcf4f. Anticancer Drugs. 2012. PMID: 22205152
-
Bioreductive activation of mitoxantrone by NADPH cytochrome P450 reductase. Implications for increasing its ability to inhibit the growth of sensitive and multidrug resistant leukaemia HL60 cells.Cancer Lett. 2007 Jan 8;245(1-2):252-62. doi: 10.1016/j.canlet.2006.01.012. Epub 2006 Mar 29. Cancer Lett. 2007. PMID: 16574318
-
Multifunctional micelle delivery system for overcoming multidrug resistance of doxorubicin.J Drug Target. 2018 Apr;26(4):289-295. doi: 10.1080/1061186X.2017.1379525. Epub 2017 Sep 21. J Drug Target. 2018. PMID: 28901798 Review.
Cited by
-
Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration.Life (Basel). 2020 Aug 24;10(9):162. doi: 10.3390/life10090162. Life (Basel). 2020. PMID: 32846924 Free PMC article.
-
A genistein derivative, ITB-301, induces microtubule depolymerization and mitotic arrest in multidrug-resistant ovarian cancer.Cancer Chemother Pharmacol. 2011 Oct;68(4):1033-44. doi: 10.1007/s00280-011-1575-2. Epub 2011 Feb 22. Cancer Chemother Pharmacol. 2011. PMID: 21340606 Free PMC article.
-
Doxorubicin-conjugated dexamethasone induced MCF-7 apoptosis without entering the nucleus and able to overcome MDR-1-induced resistance.Drug Des Devel Ther. 2018 Aug 1;12:2361-2369. doi: 10.2147/DDDT.S168588. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30122894 Free PMC article.
-
Magnetic Driven Nanocarriers for pH-Responsive Doxorubicin Release in Cancer Therapy.Molecules. 2020 Jan 14;25(2):333. doi: 10.3390/molecules25020333. Molecules. 2020. PMID: 31947577 Free PMC article.
-
Mechanisms of Doxorubicin Toxicity in Pancreatic β-Cells.Toxicol Sci. 2016 Aug;152(2):395-405. doi: 10.1093/toxsci/kfw096. Epub 2016 Jun 2. Toxicol Sci. 2016. PMID: 27255381 Free PMC article.
References
-
- Adams GE, Stratford IJ (1994) Bioreductive drugs for cancer therapy: the search for tumor specificity. Int J Radiat Oncol Biol Phys 29: 231–238 - PubMed
-
- Bailey SM, Lewis AD, Patterson LH, Fisher GR, Knox RJ, Workman P (2001) Involvement of NADPH: cytochrome P450 reductase in the activation of indoloquinone EO9 to free radical and DNA damaging species. Biochem Pharmacol 62: 461–468 - PubMed
-
- Bartoszek A (2002) Metabolic activation of adriamycin by NADPH-cytochrome P450 reductase; overview of its biological and biochemical effects. Acta Biochim Pol 49: 323–331 - PubMed
-
- Bartoszek A, Pawlowska J, Tarasiuk J (1999) Adriamycin metabolism mediated by NADPH cytochrome P-450 reductase: NADPH consumption and radical formation. 7th International Symposium on Molecular Aspects of Chemotherapy, Gdansk, 8–11 September 1999, Poland, Abstract Book, p. 90
-
- Bartoszek A, Wolf CR (1992) Enhancement of doxorubicin toxicity following activation by NADPH cytohrome P450 reductase. Biochem Pharmacol 43: 1449–1457 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

