Antibody avidity maturation during severe acute respiratory syndrome-associated coronavirus infection
- PMID: 15942907
- PMCID: PMC7109913
- DOI: 10.1086/430615
Antibody avidity maturation during severe acute respiratory syndrome-associated coronavirus infection
Abstract
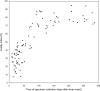
The maturation of virus-specific immunoglobulin G avidity during severe acute respiratory syndrome-associated coronavirus infection was examined. The avidity indices were low (mean +/- SD, 30.8% +/- 11.6%) among serum samples collected < or =50 days after fever onset, intermediate (mean +/- SD, 52.1% +/- 14.1%) among samples collected between days 51 and 90, and high (mean +/- SD, 78.1% +/- 8.0%) among samples collected after day 90. Avidity indices of 40% and 55% could be considered as cutoff values for determination of recent (< or =50 days) and past (>65 days) infection, respectively. Measurement of antibody avidity can be used to differentiate primary infection from reexposure and to assess humoral responses to candidate vaccines.
Figures
Similar articles
-
Use of antibody avidity assays for diagnosis of severe acute respiratory syndrome coronavirus infection.Clin Vaccine Immunol. 2007 Nov;14(11):1433-6. doi: 10.1128/CVI.00056-07. Epub 2007 Sep 19. Clin Vaccine Immunol. 2007. PMID: 17881505 Free PMC article.
-
Longitudinal profiles of immunoglobulin G antibodies against severe acute respiratory syndrome coronavirus components and neutralizing activities in recovered patients.Scand J Infect Dis. 2011 Jul;43(6-7):515-21. doi: 10.3109/00365548.2011.560184. Epub 2011 Mar 2. Scand J Infect Dis. 2011. PMID: 21366405
-
Natural course of severe acute respiratory syndrome-associated coronavirus immunoglobulin after infection.J Infect Dis. 2004 Nov 1;190(9):1706-7; author reply 1707. doi: 10.1086/424573. J Infect Dis. 2004. PMID: 15478079 Free PMC article. No abstract available.
-
Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus.Clin Microbiol Infect. 2004 Dec;10(12):1062-6. doi: 10.1111/j.1469-0691.2004.01009.x. Clin Microbiol Infect. 2004. PMID: 15606632 Free PMC article.
-
SARS Immunity and Vaccination.Cell Mol Immunol. 2004 Jun;1(3):193-8. Cell Mol Immunol. 2004. PMID: 16219167 Review.
Cited by
-
Rapid, sensitive, and species-independent detection of Crimean Congo hemorrhagic fever virus nucleoprotein and GP38 antibodies.EBioMedicine. 2025 Aug;118:105857. doi: 10.1016/j.ebiom.2025.105857. Epub 2025 Jul 23. EBioMedicine. 2025. PMID: 40706445 Free PMC article.
-
TOP-Plus Is a Versatile Biosensor Platform for Monitoring SARS-CoV-2 Antibody Durability.Clin Chem. 2021 Sep 1;67(9):1249-1258. doi: 10.1093/clinchem/hvab069. Clin Chem. 2021. PMID: 33914041 Free PMC article.
-
The variability of the serological response to SARS-corona virus-2: Potential resolution of ambiguity through determination of avidity (functional affinity).J Med Virol. 2021 Jan;93(1):311-322. doi: 10.1002/jmv.26262. Epub 2020 Jul 15. J Med Virol. 2021. PMID: 32633840 Free PMC article. Review.
-
Oral Immunization with Escherichia coli Nissle 1917 Expressing SARS-CoV-2 Spike Protein Induces Mucosal and Systemic Antibody Responses in Mice.Biomolecules. 2023 Mar 21;13(3):569. doi: 10.3390/biom13030569. Biomolecules. 2023. PMID: 36979504 Free PMC article.
-
The COVID-19 second wave: A perspective to be explored.Braz J Infect Dis. 2021 Jan-Feb;25(1):101537. doi: 10.1016/j.bjid.2020.101537. Epub 2020 Dec 26. Braz J Infect Dis. 2021. PMID: 33378685 Free PMC article. No abstract available.
References
-
- World Health Organization Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available at: http://www.who.int/csr/sars/country/table2003_09_23/en/print.html. Accessed 30 December 2003.
-
- Lim PL, Kurup A, Gopalakrishna G, et al. Laboratory‐acquired severe acute respiratory syndrome. N Engl J Med. 2004;350:1740–5. - PubMed
-
- World Health Organization Severe acute respiratory syndrome (SARS) in Taiwan, China. Available at: http://www.who.int/csr/don/2003_12_17/en/. Accessed 5 August 2004.
-
- World Health Organization Update 4: review of probable and laboratory‐confirmed SARS cases in southern China. Available at: http://www.who.int/csr/don/2004_01_27/en/. Accessed 5 August 2004.
-
- World Health Organization Investigation into China’s recent SARS outbreak yields important lessons for global public health. Available at: http://www.wpro.who.int/sars/docs/update/update_07022004.asp. Accessed 5 August 2004.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous