Mutating His29, His125, His133 or His158 abolishes glycosylphosphatidylinositol-specific phospholipase D catalytic activity
- PMID: 15943582
- PMCID: PMC1276926
- DOI: 10.1042/BJ20050656
Mutating His29, His125, His133 or His158 abolishes glycosylphosphatidylinositol-specific phospholipase D catalytic activity
Abstract
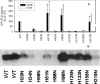
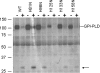
Glycosylphosphatidylinositol (GPI)-specific phospholipase D (GPI-PLD) specifically cleaves GPIs. This phospholipase D is a secreted protein consisting of two domains: an N-terminal catalytic domain and a predicted C-terminal b-propeller. Although the biochemical properties of GPI-PLD have been extensively studied, its catalytic site has not been identified. We hypothesized that a histidine residue(s) may play a critical role in the catalytic activity of GPI-PLD, based on the observations that (i) Zn2+, which utilizes histidine residues for binding, is required for GPI-PLD catalytic activity, (ii) a phosphohistidine intermediate is involved in phospholipase D hydrolysis of phosphatidylcholine, (iii) computer modelling suggests a catalytic site containing histidine residues, and (iv) our observation that diethyl pyrocarbonate, which modifies histidine residues, inhibits GPI-PLD catalytic activity. Individual mutation of the ten histidine residues to asparagine in the catalytic domain of murine GPI-PLD resulted in three general phenotypes: not secreted or retained (His56 or His88), secreted with catalytic activity (His34, His81, His98 or His219) and secreted without catalytic activity (His29, His125, His133 or His158). Changing His133 but not His29, His125 or His158 to Cys resulted in a mutant that retained catalytic activity, suggesting that at least His133 is involved in Zn2+ binding. His133 and His158 also retained the biochemical properties of wild-type GPI-PLD including trypsin cleavage pattern and phosphorylation by protein kinase A. Hence, His29, His125, His133 and His158 are required for GPI-PLD catalytic activity.
Figures



Similar articles
-
Midportion antibodies stimulate glycosylphosphatidylinositol-specific phospholipase D activity.Arch Biochem Biophys. 1999 Oct 15;370(2):278-84. doi: 10.1006/abbi.1999.1400. Arch Biochem Biophys. 1999. PMID: 10510287
-
In vitro phosphorylation of purified glycosylphosphatidylinositol-specific phospholipase D.Biol Chem. 1999 May;380(5):585-8. doi: 10.1515/BC.1999.074. Biol Chem. 1999. PMID: 10384965
-
A distant evolutionary relationship between GPI-specific phospholipase D and bacterial phosphatidylcholine-preferring phospholipase C.FEBS Lett. 2004 Jul 2;569(1-3):229-34. doi: 10.1016/j.febslet.2004.05.071. FEBS Lett. 2004. PMID: 15225639
-
The PLD superfamily: insights into catalysis.Biochim Biophys Acta. 1999 Jul 30;1439(2):187-97. doi: 10.1016/s1388-1981(99)00094-3. Biochim Biophys Acta. 1999. PMID: 10425395 Review.
-
Phospholipase D as a catalyst: application in phospholipid synthesis, molecular structure and protein engineering.J Biosci Bioeng. 2013 Sep;116(3):271-80. doi: 10.1016/j.jbiosc.2013.03.008. Epub 2013 Apr 29. J Biosci Bioeng. 2013. PMID: 23639419 Review.
Cited by
-
GPI-AP release in cellular, developmental, and reproductive biology.J Lipid Res. 2016 Apr;57(4):538-45. doi: 10.1194/jlr.R063032. Epub 2015 Nov 22. J Lipid Res. 2016. PMID: 26593072 Free PMC article. Review.
-
Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain.Science. 2020 Jul 10;369(6500):167-173. doi: 10.1126/science.aaw2622. Science. 2020. PMID: 32646997 Free PMC article.
-
Phospholipase D: enzymology, functionality, and chemical modulation.Chem Rev. 2011 Oct 12;111(10):6064-119. doi: 10.1021/cr200296t. Epub 2011 Sep 22. Chem Rev. 2011. PMID: 21936578 Free PMC article. Review. No abstract available.
-
The wooly mutation (wly) on mouse chromosome 11 is associated with a genetic defect in Fam83g.BMC Res Notes. 2013 May 9;6:189. doi: 10.1186/1756-0500-6-189. BMC Res Notes. 2013. PMID: 23656696 Free PMC article.
-
GPLD1 Attenuates Heart Failure via Dual-Membrane Localization to Inhibit uPAR.Circ Res. 2025 Aug 15;137(5):e124-e143. doi: 10.1161/CIRCRESAHA.124.325623. Epub 2025 Jul 9. Circ Res. 2025. PMID: 40631685
References
-
- Low M. G., Brodbeck U. Enzymes cleaving the phosphodiester bond in the GPI anchor. In: Hoessli D. C., Ilangumaran S., editors. GPI-anchored Membrane Proteins and Carbohydrates. Austin: R.G. Landes; 1999. pp. 167–186.
-
- Low M. G. Structure and function of GPI-specific phospholipases. In: Young N. G., Moss J., editors. PNH and the GPI-linked Proteins. San Diego, CA: Academic Press; 2000. pp. 239–268.
-
- Stadelmann B., Zurbiggen A., Brodbeck U. Distribution of glycosylphosphatidylinositol-specific phospholipase D mRNA in bovine tissue sections. Cell Tissue Res. 1993;274:547–552. - PubMed
-
- LeBoeuf R. C., Caldwell M., Guo Y., Metz C., Davitz M. A., Olson L. K., Deeg M. A. Mouse glycosylphosphatidylinositol-specific phospholipase D (Gpld1) characterization. Mam. Gen. 1998;9:710–714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

