Localization of plasma membrane t-SNAREs syntaxin 2 and 3 in intracellular compartments
- PMID: 15943887
- PMCID: PMC1156879
- DOI: 10.1186/1471-2121-6-26
Localization of plasma membrane t-SNAREs syntaxin 2 and 3 in intracellular compartments
Abstract
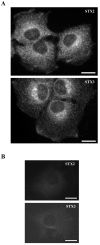
Background: Membrane fusion requires the formation of a complex between a vesicle protein (v-SNARE) and the target membrane proteins (t-SNAREs). Syntaxin 2 and 3 are t-SNAREs that, according to previous over-expression studies, are predominantly localized at the plasma membrane. In the present study we investigated localization of the endogenous syntaxin 2 and 3.
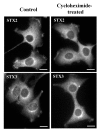
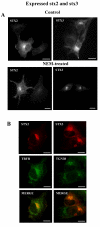
Results: Endogenous syntaxin 2 and 3 were found in NRK cells in intracellular vesicular structures in addition to regions of the plasma membrane. Treatment of these cells with N-ethylmaleimide (NEM), which is known to inactivate membrane fusion, caused syntaxin 3 to accumulate in the trans-Golgi network and syntaxin 2 in perinuclear membrane vesicles. Kinetic analysis in the presence of NEM indicated that this redistribution of syntaxin 2 and 3 takes place via actin containing structures.
Conclusion: Our data suggest that syntaxin 2 cycles between the plasma membrane and the perinuclear compartment whereas syntaxin 3 cycles between the plasma membrane and the trans-Golgi network. It is possible that this cycling has an important role in the regulation of t-SNARE function.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

