Mutant kinesin-2 motor subunits increase chromosome loss
- PMID: 15944218
- PMCID: PMC1182318
- DOI: 10.1091/mbc.e05-05-0404
Mutant kinesin-2 motor subunits increase chromosome loss
Abstract
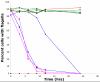
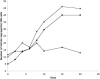
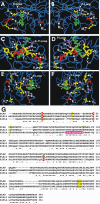
The Chlamydomonas anterograde intraflagellar transport motor, kinesin-2, is isolated as a heterotrimeric complex containing two motor subunits and a nonmotor subunit known as kinesin-associated polypeptide or KAP. One of the two motor subunits is encoded by the FLA10 gene. The sequence of the second motor subunit was obtained by mass spectrometry and sequencing. It shows 46.9% identity with the Fla10 motor subunit and the gene maps to linkage group XII/XIII near RPL9. The temperature-sensitive flagellar assembly mutants fla1 and fla8 are linked to this kinesin-2 motor subunit. In each strain, a unique single point mutation gives rise to a unique single amino acid substitution within the motor domain. The fla8 strain is named fla8-1 and the fla1 strain is named fla8-2. The fla8 and fla10 alleles show a chromosome loss phenotype. To analyze this chromosome loss phenotype, intragenic revertants of fla8-1, fla8-2, and fla10-14 were generated. The analysis of the mutants and the revertants demonstrates the importance of a pocket in the amino terminus of these motor subunits for both motor activity and for a novel, dominant effect on the fidelity of chromosome segregation.
Figures

Similar articles
-
The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein.J Cell Biol. 1994 Jul;126(1):175-88. doi: 10.1083/jcb.126.1.175. J Cell Biol. 1994. PMID: 8027176 Free PMC article.
-
Kinesin-II is not essential for mitosis and cell growth in Chlamydomonas.Cell Motil Cytoskeleton. 2002 Aug;52(4):195-201. doi: 10.1002/cm.10051. Cell Motil Cytoskeleton. 2002. PMID: 12112134
-
Kinesin-2 from C. reinhardtii Is an Atypically Fast and Auto-inhibited Motor that Is Activated by Heterotrimerization for Intraflagellar Transport.Curr Biol. 2020 Mar 23;30(6):1160-1166.e5. doi: 10.1016/j.cub.2020.01.046. Epub 2020 Mar 5. Curr Biol. 2020. PMID: 32142698 Free PMC article.
-
Kinesin-2: a family of heterotrimeric and homodimeric motors with diverse intracellular transport functions.Annu Rev Cell Dev Biol. 2013;29:443-69. doi: 10.1146/annurev-cellbio-101512-122335. Epub 2013 Jun 3. Annu Rev Cell Dev Biol. 2013. PMID: 23750925 Review.
-
Molecular mechanisms of kinesin-14 motors in spindle assembly and chromosome segregation.J Cell Sci. 2017 Jul 1;130(13):2097-2110. doi: 10.1242/jcs.200261. J Cell Sci. 2017. PMID: 28668932 Review.
Cited by
-
The awesome power of dikaryons for studying flagella and basal bodies in Chlamydomonas reinhardtii.Cytoskeleton (Hoboken). 2014 Feb;71(2):79-94. doi: 10.1002/cm.21157. Epub 2013 Dec 12. Cytoskeleton (Hoboken). 2014. PMID: 24272949 Free PMC article. Review.
-
Getting to the heart of intraflagellar transport using Trypanosoma and Chlamydomonas models: the strength is in their differences.Cilia. 2013 Nov 29;2(1):16. doi: 10.1186/2046-2530-2-16. Cilia. 2013. PMID: 24289478 Free PMC article.
-
Mechanism of ciliary disassembly.Cell Mol Life Sci. 2016 May;73(9):1787-802. doi: 10.1007/s00018-016-2148-7. Epub 2016 Feb 11. Cell Mol Life Sci. 2016. PMID: 26869233 Free PMC article. Review.
-
Who drives the ciliary highway?Bioarchitecture. 2012 Jul-Aug;2(4):111-7. doi: 10.4161/bioa.21101. Epub 2012 Jul 1. Bioarchitecture. 2012. PMID: 22960672 Free PMC article. Review.
-
Genetic and genomic approaches to identify genes involved in flagellar assembly in Chlamydomonas reinhardtii.Methods Cell Biol. 2015;127:349-86. doi: 10.1016/bs.mcb.2014.12.001. Epub 2015 Feb 14. Methods Cell Biol. 2015. PMID: 25837400 Free PMC article.
References
-
- Asamizu, E., Miura, K., Kucho, K., Inoue, Y., Fukuzawa, H., Ohyama, K., Nakamura, Y., and Tabata, S. (2000). Generation of expressed sequence tags from low-CO2 and high-CO2 adapted cells of Chlamydomonas reinhardtii. DNA Res. 7, 305-307. - PubMed
-
- Asamizu, E., Nakamura, Y., Sato, S., Fukuzawa, H., and Tabata, S. (1999). A large scale structural analysis of cDNAs in a unicellular green alga, Chlamydomonas reinhardtii. I. Generation of 3433 non-redundant expressed sequence tags. DNA Res. 6, 369-373. - PubMed
-
- Betley, J. N., Heinrich, B., Vernos, I., Sardet, C., Prodon, F., and Deshler, J. O. (2004). Kinesin II mediates Vg1 mRNA transport in Xenopus oocytes. Curr. Biol. 14, 219-224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

