Temperature compensation of neuromuscular modulation in aplysia
- PMID: 15944231
- PMCID: PMC1564116
- DOI: 10.1152/jn.00481.2005
Temperature compensation of neuromuscular modulation in aplysia
Abstract
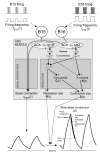
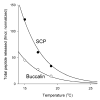
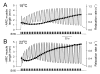
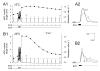
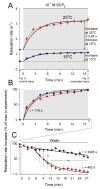
Physiological systems that must operate over a range of temperatures often incorporate temperature-compensatory mechanisms to maintain their output within a relatively narrow, functional range of values. We analyze here an example in the accessory radula closer (ARC) neuromuscular system, a representative part of the feeding neuromusculature of the sea slug Aplysia. The ARC muscle's two motor neurons, B15 and B16, release, in addition to ACh that contracts the muscle, modulatory peptide cotransmitters that, through a complex network of effects in the muscle, shape the ACh-induced contractions. It is believed that this modulation is critical in optimizing the performance of the muscle for successful, efficient feeding behavior. However, previous work has shown that the release of the modulatory peptides from the motor neurons decreases dramatically with increasing temperature. From 15 to 25 degrees C, for example, release decreases 20-fold. Yet Aplysia live and feed successfully not only at 15 degrees C, but at 25 degrees C and probably at higher temperatures. Here, working with reduced B15/B16-ARC preparations in vitro as well as a mathematical model of the system, we have found a resolution of this apparent paradox. Although modulator release decreases 20-fold when the temperature is raised from 15 to 25 degrees C, the observed modulation of contraction shape does not decrease at all. Two mechanisms are responsible. First, further downstream within the modulatory network, the modulatory effects themselves-experimentally dissected by exogenous modulator application-have temperature dependencies opposite to that of modulator release, increasing with temperature. Second, the saturating curvature of the dose-response relations within the network diminishes the downstream impact of the decrease of modulator release. Thus two quite distinct mechanisms, one depending on the characteristics of the individual components of the network and the other emerging from the network's structure, combine to compensate for temperature changes to maintain the output of this physiological system.
Figures


Comment in
-
Simple mechanism for stabilizing motor output. Focus on "temperature compensation of neuromuscular modulation in aplysia".J Neurophysiol. 2005 Nov;94(5):2997-8. doi: 10.1152/jn.00636.2005. J Neurophysiol. 2005. PMID: 16222070 No abstract available.
Similar articles
-
Modeling neuromuscular modulation in Aplysia. III. Interaction of central motor commands and peripheral modulatory state for optimal behavior.J Neurophysiol. 2005 Mar;93(3):1523-56. doi: 10.1152/jn.00475.2004. Epub 2004 Oct 6. J Neurophysiol. 2005. PMID: 15469963
-
Simple mechanism for stabilizing motor output. Focus on "temperature compensation of neuromuscular modulation in aplysia".J Neurophysiol. 2005 Nov;94(5):2997-8. doi: 10.1152/jn.00636.2005. J Neurophysiol. 2005. PMID: 16222070 No abstract available.
-
Multiple presynaptic and postsynaptic sites of inhibitory modulation by myomodulin at ARC neuromuscular junctions of Aplysia.J Neurophysiol. 2003 Mar;89(3):1488-502. doi: 10.1152/jn.00140.2002. J Neurophysiol. 2003. PMID: 12626624
-
Functional roles of peptide cotransmitters at neuromuscular synapses in Aplysia.Mol Neurobiol. 1993 Fall-Winter;7(3-4):335-47. doi: 10.1007/BF02769181. Mol Neurobiol. 1993. PMID: 8179842 Review.
-
Behavioral switching of biting and of directed head turning in Aplysia: explorations using neural network models.Acta Biol Hung. 1992;43(1-4):315-28. Acta Biol Hung. 1992. PMID: 1299121 Review. No abstract available.
Cited by
-
Growth at Cold Temperature Increases the Number of Motor Neurons to Optimize Locomotor Function.Curr Biol. 2019 Jun 3;29(11):1787-1799.e5. doi: 10.1016/j.cub.2019.04.072. Epub 2019 May 23. Curr Biol. 2019. PMID: 31130453 Free PMC article.
-
Circuit Robustness to Temperature Perturbation Is Altered by Neuromodulators.Neuron. 2018 Nov 7;100(3):609-623.e3. doi: 10.1016/j.neuron.2018.08.035. Epub 2018 Sep 20. Neuron. 2018. PMID: 30244886 Free PMC article.
-
The role of feedback and modulation in determining temperature resiliency in the lobster cardiac nervous system.Front Neurosci. 2023 Mar 9;17:1113843. doi: 10.3389/fnins.2023.1113843. eCollection 2023. Front Neurosci. 2023. PMID: 36968508 Free PMC article.
-
Phase maintenance in a rhythmic motor pattern during temperature changes in vivo.J Neurophysiol. 2014 Jun 15;111(12):2603-13. doi: 10.1152/jn.00906.2013. Epub 2014 Mar 26. J Neurophysiol. 2014. PMID: 24671541 Free PMC article.
-
Neuromodulation to the Rescue: Compensation of Temperature-Induced Breakdown of Rhythmic Motor Patterns via Extrinsic Neuromodulatory Input.PLoS Biol. 2015 Sep 29;13(9):e1002265. doi: 10.1371/journal.pbio.1002265. eCollection 2015. PLoS Biol. 2015. PMID: 26417944 Free PMC article.
References
-
- Bardales JR, Díaz-Enrich MJ, Ibarguren I, Villamarín JA. Isoforms of cAMP-dependent protein kinase in the bivalve mollusk Mytilus galloprovincialis: activation by cyclic nucleotides and effect of temperature. Arch Biochem Biophys. 2004;432:71–78. - PubMed
-
- Brezina V, Bank B, Cropper EC, Rosen S, Vilim FS, Kupfermann I, Weiss KR. Nine members of the myomodulin family of peptide cotransmitters at the B16-ARC neuromuscular junction of Aplysia. J Neurophysiol. 1995;74:54–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

