Mouse chromaffin cells have two populations of dense core vesicles
- PMID: 15944233
- PMCID: PMC12058276
- DOI: 10.1152/jn.00316.2005
Mouse chromaffin cells have two populations of dense core vesicles
Abstract
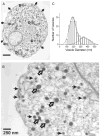
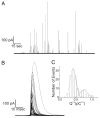
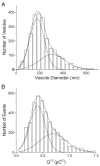
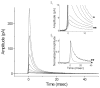
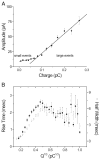
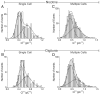
The quantal hypothesis states that neurotransmitter is released in discrete packages, quanta, thought to represent the neurotransmitter content of individual vesicles. If true, then vesicle size should influence quantal size. Although chromaffin cells are generally thought to have a single population of secretory vesicles, our electron microscopy analysis suggested two populations as the size distribution was best described as the sum of two Gaussians. The average volume difference was fivefold. To test whether this difference in volume affected quantal size, neurotransmitter release from permeabilized cells exposed to 100 microM Ca2+ was measured with amperometry. Quantal content was bimodally distributed with both large and small events; the distribution of vesicle sizes predicted by amperometry was extremely similar to those measured with electron microscopy. In addition, each population of events exhibited distinct release kinetics. These results suggest that chromaffin cells have two populations of dense core vesicles (DCV) with unique secretory properties and which may represent two distinct synthetic pathways for DCV biogenesis or alternatively they may represent different stages of biosynthesis.
Figures

References
-
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
-
- Ales E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. - PubMed
-
- Anderson Ba. A Practical Information-Theoretic Approach. New York: Springer-Verlag; 2002. Model Selection and Multimodel Inference.
-
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long- term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. - PubMed
-
- Bauer RA, Khera RS, Lieber JL, Angleson JK. Recycling of intact dense core vesicles in neurites of NGF-treated PC12 cells. FEBS Lett. 2004a;571:107–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

