Variability of swallowing performance in intact, freely feeding aplysia
- PMID: 15944235
- PMCID: PMC1224712
- DOI: 10.1152/jn.00280.2005
Variability of swallowing performance in intact, freely feeding aplysia
Erratum in
- J Neurophysiol. 2008 Oct;100(4):2453
Abstract
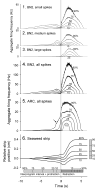
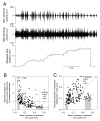
Variability in nervous systems is often taken to be merely "noise." Yet in some cases it may play a positive, active role in the production of behavior. The central pattern generator (CPG) that drives the consummatory feeding behaviors of Aplysia generates large, quasi-random variability in the parameters of the feeding motor programs from one cycle to the next; the variability then propagates through the firing patterns of the motor neurons to the contractions of the feeding muscles. We have proposed that, when the animal is faced with a new, imperfectly known feeding task in each cycle, the variability implements a trial-and-error search through the space of possible feeding movements. Although this strategy will not be successful in every cycle, over many cycles it may be the optimal strategy for feeding in an uncertain and changing environment. To play this role, however, the variability must actually appear in the feeding movements and, presumably, in the functional performance of the feeding behavior. Here we have tested this critical prediction. We have developed a technique to measure, in intact, freely feeding animals, the performance of Aplysia swallowing behavior, by continuously recording with a length transducer the movement of the seaweed strip being swallowed. Simultaneously, we have recorded with implanted electrodes activity at each of the internal levels, the CPG, motor neurons, and muscles, of the feeding neuromusculature. Statistical analysis of a large data set of these recordings suggests that functional performance is not determined strongly by one or a few parameters of the internal activity, but weakly by many. Most important, the internal variability does emerge in the behavior and its functional performance. Even when the animal is swallowing a long, perfectly regular seaweed strip, remarkably, the length swallowed from cycle to cycle is extremely variable, as variable as the parameters of the activity of the CPG, motor neurons, and muscles.
Figures







Similar articles
-
Tight or loose coupling between components of the feeding neuromusculature of Aplysia?J Neurophysiol. 2005 Jul;94(1):531-49. doi: 10.1152/jn.01338.2004. Epub 2005 May 25. J Neurophysiol. 2005. PMID: 15917315
-
Cycle-to-cycle variability of neuromuscular activity in Aplysia feeding behavior.J Neurophysiol. 2004 Jul;92(1):157-80. doi: 10.1152/jn.01190.2003. Epub 2004 Feb 25. J Neurophysiol. 2004. PMID: 14985412
-
Modeling neuromuscular modulation in Aplysia. III. Interaction of central motor commands and peripheral modulatory state for optimal behavior.J Neurophysiol. 2005 Mar;93(3):1523-56. doi: 10.1152/jn.00475.2004. Epub 2004 Oct 6. J Neurophysiol. 2005. PMID: 15469963
-
Feeding neural networks in the mollusc Aplysia.Neurosignals. 2004 Jan-Apr;13(1-2):70-86. doi: 10.1159/000076159. Neurosignals. 2004. PMID: 15004426 Review.
-
Premotor neurons in the feeding system of Aplysia californica.J Neurobiol. 1989 Jul;20(5):497-512. doi: 10.1002/neu.480200516. J Neurobiol. 1989. PMID: 2664083 Review.
Cited by
-
The neuromuscular transform of the lobster cardiac system explains the opposing effects of a neuromodulator on muscle output.J Neurosci. 2013 Oct 16;33(42):16565-75. doi: 10.1523/JNEUROSCI.2903-13.2013. J Neurosci. 2013. PMID: 24133260 Free PMC article.
-
Order in spontaneous behavior.PLoS One. 2007 May 16;2(5):e443. doi: 10.1371/journal.pone.0000443. PLoS One. 2007. PMID: 17505542 Free PMC article.
-
State dependence of network output: modeling and experiments.J Neurosci. 2008 Nov 12;28(46):11806-13. doi: 10.1523/JNEUROSCI.3796-08.2008. J Neurosci. 2008. PMID: 19005044 Free PMC article. Review.
-
Cycle-to-cycle variability as an optimal behavioral strategy.Neurocomputing (Amst). 2006 Jun 1;69(10-12):1120-1124. doi: 10.1016/j.neucom.2005.12.057. Neurocomputing (Amst). 2006. PMID: 19830256 Free PMC article.
-
Incorporating buccal mass planar mechanics and anatomical features improves neuromechanical modeling of Aplysia feeding behavior.Biol Cybern. 2025 Jul 7;119(4-6):17. doi: 10.1007/s00422-025-01017-1. Biol Cybern. 2025. PMID: 40622423 Free PMC article.
References
-
- Beer RD, Chiel HJ, Gallagher JC. Evolution and analysis of model CPGs for walking: II. General principles and individual variability. J Comput Neurosci. 1999;7:119–147. - PubMed
-
- Bernstein N.The Co-ordination and Regulation of Movement. Oxford: Pergamon Press, 1967.
-
- Brezina V, Horn CC, Weiss KR. Modeling neuromuscular modulation in Aplysia. III. Interaction of central motor commands and peripheral modulatory state for optimal behavior. J Neurophysiol. 2005a;93:1523–1556. - PubMed
-
- Brezina V, Orekhova IV, Weiss KR. The neuromuscular transform: the dynamic, nonlinear link between motor neuron firing patterns and muscle contraction in rhythmic behaviors. J Neurophysiol. 2000a;83:207–231. - PubMed
-
- Brezina V, Orekhova IV, Weiss KR. Optimization of rhythmic behaviors by modulation of the neuromuscular transform. J Neurophysiol. 2000b;83:260–279. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

