Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations
- PMID: 15944302
- PMCID: PMC3843132
- DOI: 10.4049/jimmunol.174.12.7961
Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations
Abstract
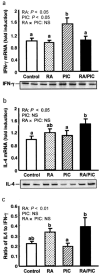
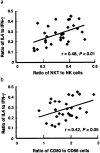
Retinoic acid (RA), a bioactive retinoid, and polyriboinosinic:polyribocytidylic acid (PIC) are known to promote immunity in vitamin A-deficient animals. In this study, we hypothesized that RA, PIC, and the combination can provide significant immunoadjuvant activity even in the vitamin A-adequate state. Six-week-old C57BL/6 mice were immunized with tetanus toxoid (TT) and treated with RA and/or PIC at priming in three independent studies of short and long duration. RA and PIC differentially regulated both primary and secondary anti-TT IgG isotypes, whereas the combination of RA + PIC stimulated the highest level of anti-TT IgG production and, concomitantly, a ratio of IgG1 to IgG2a similar to that of the control group. The regulation of Ab response was strongly associated with type 1/type 2 cytokine gene expression. Whereas RA reduced type 1 cytokines (IFN-gamma and IL-12), PIC enhanced both type 1 and type 2 cytokines (IL-4 and IL-12) and cytokine-related transcription factors. Despite the presence of PIC, the IL-4:IFN-gamma ratio was significantly elevated by RA. In addition, RA and/or PIC modulated NK/NKT cell populations and the level of expression of the costimulatory molecules CD80/CD86, evident 3 days after priming. Notably, the NKT:NK and CD80:CD86 ratios were correlated with the IL-4:IFN-gamma ratio, indicative of multiple converging modes of regulation. Overall, RA, PIC, and RA + PIC rapidly and differentially shaped the anti-tetanus Ig response. The robust, durable, and proportionate increase in all anti-TT IgG isotypes induced by RA + PIC suggests that this combination is promising as a means to enhance the Ab response to TT and similar vaccines.
Conflict of interest statement
Figures


References
-
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. - PubMed
-
- West KP., Jr Extent of vitamin A deficiency among preschool children and women of reproductive age. J Nutr. 2002;132:2857S–2866S. - PubMed
-
- Van DE, Kulier R, Gulmezoglu AM, Villar J. Vitamin A supplementation during pregnancy. Cochrane Database Syst Rev. 2002;4:CD001996. - PubMed
-
- Semba RD. Vitamin A and infectious diseases. In: Livrea MA, editor. Vitamin A and Retinoids: An Update of Biological Aspects and Clinical Applications. Birkhauser Verlag Basel; Switzerland: 2000. pp. 97–108.
-
- Ramakrishnan U, Martorell R. The role of vitamin A in reducing child mortality and morbidity and improving growth. Salud Publica Mex. 1998;40:189–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

