Multiple roles of a heterotrimeric G-protein gamma-subunit in governing growth and development of Aspergillus nidulans
- PMID: 15944346
- PMCID: PMC1456535
- DOI: 10.1534/genetics.105.042796
Multiple roles of a heterotrimeric G-protein gamma-subunit in governing growth and development of Aspergillus nidulans
Abstract
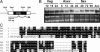
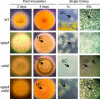
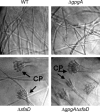
Vegetative growth signaling in the filamentous fungus Aspergillus nidulans is primarily mediated by the heterotrimeric G-protein composed of FadA (G alpha), SfaD (G beta), and a presumed G gamma. Analysis of the A. nidulans genome identified a single gene named gpgA encoding a putative G gamma-subunit. The predicted GpgA protein consists of 90 amino acids showing 72% similarity with yeast Ste18p. Deletion (delta) of gpgA resulted in restricted vegetative growth and lowered asexual sporulation. Moreover, similar to the delta sfaD mutant, the delta gpgA mutant was unable to produce sexual fruiting bodies (cleistothecia) in self-fertilization and was severely impaired with cleistothecial development in outcross, indicating that both SfaD and GpgA are required for fruiting body formation. Developmental and morphological defects caused by deletion of flbA encoding an RGS protein negatively controlling FadA-mediated vegetative growth signaling were suppressed by delta gpgA, indicating that GpgA functions in FadA-SfaD-mediated vegetative growth signaling. However, deletion of gpgA could not bypass the need for the early developmental activator FluG in asexual sporulation, suggesting that GpgA functions in a separate signaling pathway. We propose that GpgA is the only A. nidulans G gamma-subunit and is required for normal vegetative growth as well as proper asexual and sexual developmental progression.
Figures


Similar articles
-
The phosducin-like protein PhnA is required for Gbetagamma-mediated signaling for vegetative growth, developmental control, and toxin biosynthesis in Aspergillus nidulans.Eukaryot Cell. 2006 Feb;5(2):400-10. doi: 10.1128/EC.5.2.400-410.2006. Eukaryot Cell. 2006. PMID: 16467480 Free PMC article.
-
The heterotrimeric G-protein GanB(alpha)-SfaD(beta)-GpgA(gamma) is a carbon source sensor involved in early cAMP-dependent germination in Aspergillus nidulans.Genetics. 2005 Sep;171(1):71-80. doi: 10.1534/genetics.105.040584. Epub 2005 Jun 8. Genetics. 2005. PMID: 15944355 Free PMC article.
-
The Aspergillus nidulans sfaD gene encodes a G protein beta subunit that is required for normal growth and repression of sporulation.EMBO J. 1999 Oct 15;18(20):5592-600. doi: 10.1093/emboj/18.20.5592. EMBO J. 1999. PMID: 10523303 Free PMC article.
-
Heterotrimeric G protein signaling and RGSs in Aspergillus nidulans.J Microbiol. 2006 Apr;44(2):145-54. J Microbiol. 2006. PMID: 16728950 Review.
-
Genetic control of asexual sporulation in filamentous fungi.Curr Opin Microbiol. 2012 Dec;15(6):669-77. doi: 10.1016/j.mib.2012.09.006. Epub 2012 Oct 22. Curr Opin Microbiol. 2012. PMID: 23092920 Review.
Cited by
-
The putative guanine nucleotide exchange factor RicA mediates upstream signaling for growth and development in Aspergillus.Eukaryot Cell. 2012 Nov;11(11):1399-412. doi: 10.1128/EC.00255-12. Epub 2012 Sep 21. Eukaryot Cell. 2012. PMID: 23002107 Free PMC article.
-
The phosducin-like protein PhnA is required for Gbetagamma-mediated signaling for vegetative growth, developmental control, and toxin biosynthesis in Aspergillus nidulans.Eukaryot Cell. 2006 Feb;5(2):400-10. doi: 10.1128/EC.5.2.400-410.2006. Eukaryot Cell. 2006. PMID: 16467480 Free PMC article.
-
It's All in the Genes: The Regulatory Pathways of Sexual Reproduction in Filamentous Ascomycetes.Genes (Basel). 2019 Apr 30;10(5):330. doi: 10.3390/genes10050330. Genes (Basel). 2019. PMID: 31052334 Free PMC article. Review.
-
The KdmB-EcoA-RpdA-SntB chromatin complex binds regulatory genes and coordinates fungal development with mycotoxin synthesis.Nucleic Acids Res. 2022 Sep 23;50(17):9797-9813. doi: 10.1093/nar/gkac744. Nucleic Acids Res. 2022. PMID: 36095118 Free PMC article.
-
G protein γ subunit modulates expression of plant-biomass-degrading enzyme genes and mycelial-development-related genes in Penicillium oxalicum.Appl Microbiol Biotechnol. 2021 Jun;105(11):4675-4691. doi: 10.1007/s00253-021-11370-3. Epub 2021 Jun 2. Appl Microbiol Biotechnol. 2021. PMID: 34076714
References
-
- Braus, G. H., S. Krappmann and S. E. Eckert, 2002. Sexual development in Ascomycetes fruit body formation of Aspergillus nidulans, pp. 215–244 in Molecular Biology of Fungal Development, edited by H. D. Osiewacz. Marcel Dekker, New York.
-
- Cabrera-Vera, T. M., J. Vanhauwe, T. O. Thomas, M. Medkova, A. Preininger et al., 2003. Insights into G protein structure, function, and regulation. Endocr. Rev. 24 : 765–781. - PubMed
-
- Champe, S. P., D. L. Nagle and L. N. Yager, 1994. Sexual sporulation. Prog. Ind. Microbiol. 29 : 429–454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

