The heterotrimeric G-protein GanB(alpha)-SfaD(beta)-GpgA(gamma) is a carbon source sensor involved in early cAMP-dependent germination in Aspergillus nidulans
- PMID: 15944355
- PMCID: PMC1456537
- DOI: 10.1534/genetics.105.040584
The heterotrimeric G-protein GanB(alpha)-SfaD(beta)-GpgA(gamma) is a carbon source sensor involved in early cAMP-dependent germination in Aspergillus nidulans
Abstract
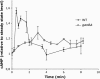
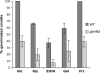
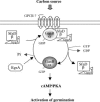
The role of heterotrimeric G-proteins in cAMP-dependent germination of conidia was investigated in the filamentous ascomycete Aspergillus nidulans. We demonstrate that the G alpha-subunit GanB mediates a rapid and transient activation of cAMP synthesis in response to glucose during the early period of germination. Moreover, deletion of individual G-protein subunits resulted in defective trehalose mobilization and altered germination kinetics, indicating that GanB(alpha)-SfaD(beta)-GpgA(gamma) constitutes a functional heterotrimer and controls cAMP/PKA signaling in response to glucose as well as conidial germination. Further genetic analyses suggest that GanB plays a primary role in cAMP/PKA signaling, whereas the SfaD-GpgA (G betagamma) heterodimer is crucial for proper activation of GanB signaling sensitized by glucose. In addition, the RGS protein RgsA is also involved in regulation of the cAMP/PKA pathway and germination via attenuation of GanB signaling. Genetic epistatic analyses led us to conclude that all controls exerted by GanB(alpha)-SfaD(beta)-GpgA(gamma) on conidial germination are mediated through the cAMP/PKA pathway. Furthermore, GanB may function in sensing various carbon sources and subsequent activation of downstream signaling for germination.
Figures
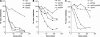
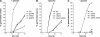
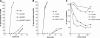
Similar articles
-
Regulators of G-protein signalling in Aspergillus nidulans: RgsA downregulates stress response and stimulates asexual sporulation through attenuation of GanB (Galpha) signalling.Mol Microbiol. 2004 Jul;53(2):529-40. doi: 10.1111/j.1365-2958.2004.04163.x. Mol Microbiol. 2004. PMID: 15228532
-
Multiple roles of a heterotrimeric G-protein gamma-subunit in governing growth and development of Aspergillus nidulans.Genetics. 2005 Sep;171(1):81-9. doi: 10.1534/genetics.105.042796. Epub 2005 Jun 8. Genetics. 2005. PMID: 15944346 Free PMC article.
-
The GanB Galpha-protein negatively regulates asexual sporulation and plays a positive role in conidial germination in Aspergillus nidulans.Genetics. 2004 Jul;167(3):1305-15. doi: 10.1534/genetics.103.025379. Genetics. 2004. PMID: 15280244 Free PMC article.
-
Heterotrimeric G protein signaling and RGSs in Aspergillus nidulans.J Microbiol. 2006 Apr;44(2):145-54. J Microbiol. 2006. PMID: 16728950 Review.
-
Translating G protein subunit diversity into functional specificity.Curr Opin Cell Biol. 2004 Apr;16(2):206-9. doi: 10.1016/j.ceb.2004.02.007. Curr Opin Cell Biol. 2004. PMID: 15196565 Review.
Cited by
-
Characterization of gprK Encoding a Putative Hybrid G-Protein-Coupled Receptor in Aspergillus fumigatus.PLoS One. 2016 Sep 1;11(9):e0161312. doi: 10.1371/journal.pone.0161312. eCollection 2016. PLoS One. 2016. PMID: 27584150 Free PMC article.
-
The pkaB gene encoding the secondary protein kinase A catalytic subunit has a synthetic lethal interaction with pkaA and plays overlapping and opposite roles in Aspergillus nidulans.Eukaryot Cell. 2005 Aug;4(8):1465-76. doi: 10.1128/EC.4.8.1465-1476.2005. Eukaryot Cell. 2005. PMID: 16087751 Free PMC article.
-
Studies of Cellulose and Starch Utilization and the Regulatory Mechanisms of Related Enzymes in Fungi.Polymers (Basel). 2020 Mar 2;12(3):530. doi: 10.3390/polym12030530. Polymers (Basel). 2020. PMID: 32121667 Free PMC article. Review.
-
Aspergillus nidulans protein kinase A plays an important role in cellulase production.Biotechnol Biofuels. 2015 Dec 18;8:213. doi: 10.1186/s13068-015-0401-1. eCollection 2015. Biotechnol Biofuels. 2015. PMID: 26690721 Free PMC article.
-
Analysis of the phosphorylome of trichoderma reesei cultivated on sugarcane bagasse suggests post-translational regulation of the secreted glycosyl hydrolase Cel7A.Biotechnol Rep (Amst). 2021 Jun 22;31:e00652. doi: 10.1016/j.btre.2021.e00652. eCollection 2021 Sep. Biotechnol Rep (Amst). 2021. PMID: 34258241 Free PMC article.
References
-
- Chen, C., and M. B. Dickman, 2005. cAMP blocks MAPK activation and sclerotial development via Rap-1 in a PKA-independent manner in Sclerotinia sclerotiorum. Mol. Microbiol. 55: 299–311. - PubMed
-
- d'Enfert, C., 1997. Fungal spore germination: insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet. Biol. 21: 163–172.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

