Differential response of Period 1 expression within the suprachiasmatic nucleus
- PMID: 15944376
- PMCID: PMC6724974
- DOI: 10.1523/JNEUROSCI.0889-05.2005
Differential response of Period 1 expression within the suprachiasmatic nucleus
Abstract
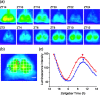
The suprachiasmatic nuclei (SCNs) of the hypothalamus contain a circadian clock that exerts profound control over rhythmic physiology and behavior. The clock consists of multiple autonomous cellular pacemakers distributed throughout the rat SCN. In response to a shift in the light schedule, the SCN rapidly changes phase to achieve the appropriate phase relationship with the shifted light schedule. Through use of a transgenic rat in which rhythmicity in transcription of the Period 1 gene was measured with a luciferase reporter (Per1-luc), we have been successful in tracking the time course of molecular rhythm phase readjustments in different regions of the SCN that occur in response to a shift in the light schedule. We find that different regions of the SCN phase adjust at different rates, leading to transient internal desynchrony in Per1-luc expression among SCN regions. This desynchrony among regions is most pronounced and prolonged when the light schedule is advanced compared with light schedule delays. A similar asymmetry in the speed of phase resetting is observed with locomotor behavior, suggesting that phase shifting kinetics within the SCN may underlay the differences observed in behavioral resetting to advances or delays in the light schedule.
Figures



References
-
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S (2002) Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol 61: 26-34. - PubMed
-
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S (1999) Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci 19: 1115-1121. - PMC - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC (1997) A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91: 1055-1064. - PubMed
-
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC (2001) MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms 16: 100-104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
