Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area
- PMID: 15944383
- PMCID: PMC6724971
- DOI: 10.1523/JNEUROSCI.0345-05.2005
Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area
Abstract
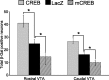
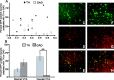
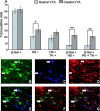
The transcription factor cAMP response element binding protein (CREB) is implicated in the actions of drugs of abuse in several brain areas, but little information is available about a role for CREB in the ventral tegmental area (VTA), one of the key reward regions of the brain. Here, we demonstrate that chronic exposure to drugs of abuse induces CREB activity throughout the VTA. Using viral-mediated gene transfer, we expressed green fluorescent protein (GFP)-tagged CREB or mCREB (a dominant-negative form of CREB) in the VTA and, using a conditioned place-preference paradigm, found that CREB activation within the rostral versus caudal subregions of the VTA produces opposite effects on drug reward. We identified VTA subregion-specific differences in the proportion of dopaminergic and GABAergic neurons and in the dopaminergic projections to the nucleus accumbens, another brain region implicated in drug reward, and suggest that this may contribute to behavioral differences in this study. We also measured expression levels of tyrosine hydroxylase and the AMPA glutamate receptor subunit GluR1, both of which are known to contribute to drug reward in the VTA, and found that both of these genes are upregulated following the expression of CREB-GFP and downregulated following expression of mCREB-GFP, raising the possibility that CREB may exert its effects on drug reward, in part, via regulation of these genes. These results suggest a novel role for CREB in mediating drug-induced plasticity in the VTA and establish two functionally distinct subregions of the VTA in which CREB differentially regulates drug reward.
Figures
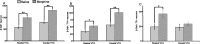



Similar articles
-
Differential distribution of CREB in the mesolimbic dopamine reward pathway.J Neurochem. 2003 Dec;87(5):1237-44. doi: 10.1046/j.1471-4159.2003.02090.x. J Neurochem. 2003. PMID: 14622103
-
Modulatory role of the intra-accumbal CB1 receptor in protein level of the c-fos and pCREB/CREB ratio in the nucleus accumbens and ventral tegmental area in extinction and morphine seeking in the rats.Brain Res Bull. 2018 Sep;142:320-327. doi: 10.1016/j.brainresbull.2018.08.017. Epub 2018 Aug 28. Brain Res Bull. 2018. PMID: 30170186
-
Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1.J Neurosci. 2000 Mar 1;20(5):RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. J Neurosci. 2000. PMID: 10684909 Free PMC article.
-
The antero-posterior heterogeneity of the ventral tegmental area.Neuroscience. 2014 Dec 12;282:198-216. doi: 10.1016/j.neuroscience.2014.09.025. Epub 2014 Sep 18. Neuroscience. 2014. PMID: 25241061 Review.
-
Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".Brain Res. 2016 Aug 15;1645:71-4. doi: 10.1016/j.brainres.2015.12.039. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740398 Free PMC article. Review.
Cited by
-
Cyr61 Promotes Inflammation of a Gouty Arthritis Model in Rats.Mediators Inflamm. 2020 Jul 24;2020:8298615. doi: 10.1155/2020/8298615. eCollection 2020. Mediators Inflamm. 2020. PMID: 32774151 Free PMC article.
-
Electron microscopic localization of corticotropin-releasing factor (CRF) and CRF receptor in rat and mouse central nucleus of the amygdala.J Comp Neurol. 2009 Jan 20;512(3):323-35. doi: 10.1002/cne.21884. J Comp Neurol. 2009. PMID: 19003957 Free PMC article.
-
The rewarding and locomotor-sensitizing effects of repeated cocaine administration are distinct and separable in mice.Neuropharmacology. 2012 Mar;62(4):1858-66. doi: 10.1016/j.neuropharm.2011.12.011. Epub 2011 Dec 16. Neuropharmacology. 2012. PMID: 22197517 Free PMC article.
-
Granulocyte-Colony-Stimulating Factor Alters the Proteomic Landscape of the Ventral Tegmental Area.Proteomes. 2018 Sep 23;6(4):35. doi: 10.3390/proteomes6040035. Proteomes. 2018. PMID: 30249060 Free PMC article.
-
Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat.Neuroscience. 2008 Apr 9;152(4):1024-31. doi: 10.1016/j.neuroscience.2008.01.046. Epub 2008 Feb 7. Neuroscience. 2008. PMID: 18355970 Free PMC article.
References
-
- Aghajanian GK, Bunney BS (1977) Dopamine “autoreceptors”: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol 297: 1-7. - PubMed
-
- Arnt J, Scheel-Kruger J (1979) GABA in the ventral tegmental area: differential regional effects on locomotion, aggression and food intake after microinjection of GABA agonists and antagonists. Life Sci 25: 1351-1360. - PubMed
-
- Bannon MJ, Michaud RL, Roth RH (1981) Mesocortical dopamine neurons. Lack of autoreceptors modulating dopamine synthesis. Mol Pharmacol 19: 270-275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
