Bidirectional regulation of cytoplasmic polyadenylation element-binding protein phosphorylation by Ca2+/calmodulin-dependent protein kinase II and protein phosphatase 1 during hippocampal long-term potentiation
- PMID: 15944388
- PMCID: PMC6724975
- DOI: 10.1523/JNEUROSCI.5051-04.2005
Bidirectional regulation of cytoplasmic polyadenylation element-binding protein phosphorylation by Ca2+/calmodulin-dependent protein kinase II and protein phosphatase 1 during hippocampal long-term potentiation
Abstract
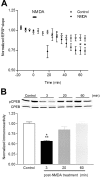
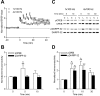
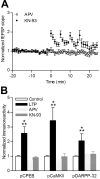
Induction of hippocampal long-term potentiation (LTP) requires activation of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII), whereas maintenance of LTP additionally requires protein synthesis. We recently reported that CaMKII stimulates protein synthesis in depolarized hippocampal neurons through phosphorylation of the mRNA translation factor cytoplasmic polyadenylation element-binding protein (CPEB), and this phosphorylation is rapidly reversed by protein phosphatase 1 (PP1). Protein synthesis-dependent late-phase LTP (L-LTP) in the hippocampus requires calcium influx through the NMDA-type glutamate receptor (NMDA-R) to activate CaMKII as well as concomitant inhibition of PP1 mediated by protein kinase A. Therefore, we investigated the regulation of CPEB phosphorylation during L-LTP. Pharmacological stimulation of the NMDA-R in hippocampal slices to produce chemical long-term depression induced a brief dephosphorylation of CPEB. Modest LTP induction (once at 100 Hz), which induces a protein synthesis-independent early-phase LTP (E-LTP), resulted in a transient phosphorylation of CPEB. However, stronger stimulation (four times at 100 Hz), known to induce protein synthesis-dependent L-LTP, elicited a prolonged phosphorylation of CPEB. Furthermore, CPEB phosphorylation correlated with phosphorylation of PP1 inhibitor dopamine- and cAMP-regulated phosphoprotein, a known substrate for protein kinase A. These results evoke the hypothesis that bidirectional regulation of CPEB phosphorylation by CaMKII and protein phosphatases may serve as a mechanism to convert E-LTP into protein synthesis-dependent L-LTP by stimulating protein synthesis and thereby stabilizing synaptic enhancement.
Figures

References
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR (1997) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276: 2042-2045. - PubMed
-
- Benson DL (1997) Dendritic compartmentation of NMDA receptor mRNA in cultured hippocampal neurons. NeuroReport 8: 823-828. - PubMed
-
- Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM (1995) Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron 15: 1403-1414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
