Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling
- PMID: 15944737
- PMCID: PMC1173141
- DOI: 10.1038/sj.emboj.7600688
Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling
Abstract
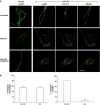
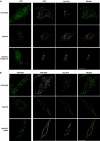
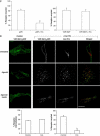
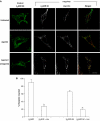
Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) is well known to terminate cell signaling by sorting activated receptors to the MVB/lysosomal pathway. Here we identify a distinct role of Hrs in promoting rapid recycling of endocytosed signaling receptors to the plasma membrane. This function of Hrs is specific for receptors that recycle in a sequence-directed manner, in contrast to default recycling by bulk membrane flow, and is distinguishable in several ways from previously identified membrane-trafficking functions of Hrs/Vps27p. In particular, Hrs function in sequence-directed recycling does not require other mammalian Class E gene products involved in MVB/lysosomal sorting, nor is receptor ubiquitination required. Mutational studies suggest that the VHS domain of Hrs plays an important role in sequence-directed recycling. Disrupting Hrs-dependent recycling prevented functional resensitization of the beta(2)-adrenergic receptor, converting the temporal profile of cell signaling by this prototypic G protein-coupled receptor from sustained to transient. These studies identify a novel function of Hrs in a cargo-specific recycling mechanism, which is critical to controlling functional activity of the largest known family of signaling receptors.
Figures

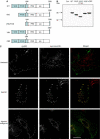
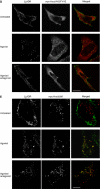
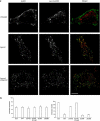
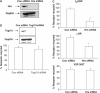
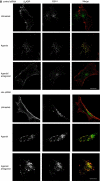
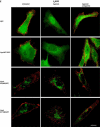
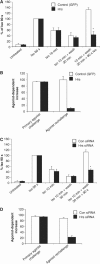
References
-
- Babst M, Odorizzi G, Estepa EJ, Emr SD (2000) Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1: 248–258 - PubMed
-
- Bache KG, Raiborg C, Mehlum A, Stenmark H (2003) STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem 278: 12513–12521 - PubMed
-
- Bilodeau PS, Urbanowski JL, Winistorfer SC, Piper RC (2002) The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat Cell Biol 4: 534–539 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

