Maintenance of long-term tumour-specific T-cell memory by residual dormant tumour cells
- PMID: 15946250
- PMCID: PMC1782166
- DOI: 10.1111/j.1365-2567.2005.02163.x
Maintenance of long-term tumour-specific T-cell memory by residual dormant tumour cells
Abstract
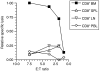
LacZ (Gal)-reactive immune cells were transferred into athymic nu/nu mice inoculated with Gal-expressing syngeneic tumour cells (ESbL-Gal) in order to study tumour-protective T-cell memory. This transfer prevented tumour outgrowth in recipients and resulted in the persistence of a high frequency of Gal-specific CD8(+) T cells in the bone marrow and spleen. In contrast, such Ag-specific memory CD8(+) T cells were not detectable by peptide-major histocompatibility complex (MHC) multimer staining in animals that had not previously received an antigenic challenge. Even though CD44(hi) memory T cells from the bone marrow showed a significantly higher turnover rate, as judged by bromodeoxyuridine (BrdU) incorporation, than respective cells from spleen or lymph nodes, as well as in comparison to CD44(lo) naïve T cells, these findings suggest that tumour-associated antigen (TAA) from residual dormant tumour cells are implicated in maintaining high frequencies of long-term surviving Gal-specific memory CD8(+) T cells. Memory T cells could be recruited to the peritoneal cavity by tumour vaccination of immunoprotected nu/nu mice and exhibited ex vivo antitumour reactivity. Long-term immune memory and tumour protection could be maintained over four successive transfers between tumour-inoculated recipients, which involved periodic antigenic restimulation in vivo prior to reisolating the cells for adoptive transfer. Using a cell line (ESbL-Gal-BM) that was established from dormant tumour cells isolated from the bone marrow of immunoprotected animals, it could be demonstrated that the tumour cells had up-regulated the expression of MHC class I molecules and down-regulated the expression of several adhesion molecules during the in vivo passage. Our results suggest that the bone marrow microenvironment has special features that are of importance for the maintenance of tumour dormancy and immunological T-cell memory, and that a low level of persisting antigen favours the maintenance of Ag-specific memory T cells over irrelevant memory T cells.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

