Circulating anti-retinal antibodies as immune markers in age-related macular degeneration
- PMID: 15946260
- PMCID: PMC1782158
- DOI: 10.1111/j.1365-2567.2005.02173.x
Circulating anti-retinal antibodies as immune markers in age-related macular degeneration
Abstract
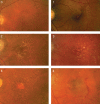
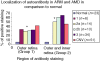
Age-related macular maculopathy (ARM) and age-related macular degeneration (AMD) are the leading causes of blindness in the Western world. Despite the magnitude of this clinical problem, very little is known about the pathogenesis of the disease. In this study, we analysed the sera (using indirect immunohistochemistry and Western blot analysis) from a very large cohort of such patients and normal age-matched controls to detect circulating anti-retinal antibodies. Patients with bilateral drusen (n = 64) and with chorioretinal neovascularization (CNV) (n = 51) were recruited in addition to age-matched control subjects (n = 39). The sera were analysed for anti-retinal immunoglobulins on retinal sections. The data were then correlated with the clinical features graded according to the International Classification and Grading System of ARM and AMD. The sera of patients with drusen (93.75%) and CNV (82.27%) were found to have a significantly (P = 0.02) higher titre of autoantibodies to the retina in comparison with controls (8.69%), indicating significant evidence of involvement of the immune process in early stages of AMD. Subsequent statistical analysis of the drusen group showed significant progressive staining (P = 0.0009) in the nuclei layers from early to late stages of ARM. Western blotting confirmed the presence of anti-retinal immunoglobulins to retinal antigens. As anti-retinal immunoglobulins are present in patients with bilateral drusen and exudative AMD, these antibodies could play a significant role in the pathogenesis of AMD. Whilst we do not have evidence that these antibodies precede disease onset, the possibility that their presence might contribute to disease progression needs to be investigated. Finally, the eventual identification of the target antigens detected by these antibodies may permit the future development of new diagnostic methods for ARM and AMD.
Figures



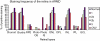
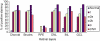
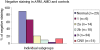
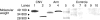

References
-
- Owen CG, Fletcher AE, Donoghue M, Rudnicka AR. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol. 2003;87:312–7. 10.1136/bjo.87.3.312. - DOI - PMC - PubMed
-
- la Cour M, Kiilgaard JF, Nissen MH. Age-related macular degeneration: epidemiology and optimal treatment. Drugs Aging. 2002;19:101–33. - PubMed
-
- Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE–Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. 10.1016/S1350-9462(01)00010-6. - DOI - PubMed
-
- Chen H, Wu L, Pan S, Wu DZ. An immunologic study on age-related macular degeneration. Yan Ke Xue Bao. 1993;9:113–20. - PubMed
-
- Gurne DH, Tso MO, Edward DP, Ripps H. Antiretinal antibodies in serum of patients with age-related macular degeneration. Ophthalmology. 1991;98:602–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

