The isoprenoid substrate specificity of isoprenylcysteine carboxylmethyltransferase: development of novel inhibitors
- PMID: 15946942
- PMCID: PMC3401627
- DOI: 10.1074/jbc.M504982200
The isoprenoid substrate specificity of isoprenylcysteine carboxylmethyltransferase: development of novel inhibitors
Abstract
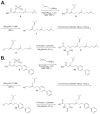
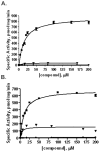
Isoprenylcysteine carboxylmethyltransferase (Icmt) is an integral membrane protein localized to the endoplasmic reticulum of eukaryotic cells that catalyzes the post-translational alpha-carboxylmethylesterification of CAAX motif proteins, including the oncoprotein Ras. Prior to methylation, these protein substrates all contain an isoprenylcysteine residue at the C terminus. In this study, we developed a variety of substrates and inhibitors of Icmt that vary in the isoprene moiety in order to gain information about the nature of the lipophilic substrate binding site. These isoprenoid-modified analogs of the minimal Icmt substrate N-acetyl-S-farnesyl-L-cysteine (AFC) were synthesized from newly and previously prepared farnesol analogs. Using both yeast and human Icmt enzymes, these compounds were found to vary widely in their ability to act as substrates, supporting the isoprenoid moiety as a key substrate recognition element for Icmt. Compound 3 is a competitive inhibitor of overexpressed yeast Icmt (K(I) = 17.1 +/- 1.7 microm). Compound 4 shows a mix of competitive and uncompetitive inhibition for both the yeast and the human Icmt proteins (yeast K(IC) = 35.4 +/- 3.4 microm, K(IU) = 614.4 +/- 148 microm; human K(IC) = 119.3 +/- 18.1 microm, K(IU) = 377.2 +/- 42.5 microm). These data further suggest that differences in substrate specificity exist between the human and yeast enzymes. Biological studies suggest that inhibition of Icmt results in Ras mislocalization and loss of cellular transformation ability, making Icmt an attractive and novel anticancer target. Further elaboration of the lead compounds synthesized and assayed here may lead to clinically useful higher potency inhibitors.
Figures

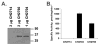




Similar articles
-
Evaluation of substrate and inhibitor binding to yeast and human isoprenylcysteine carboxyl methyltransferases (Icmts) using biotinylated benzophenone-containing photoaffinity probes.Biochem Biophys Res Commun. 2012 Jun 22;423(1):98-103. doi: 10.1016/j.bbrc.2012.05.089. Epub 2012 May 23. Biochem Biophys Res Commun. 2012. PMID: 22634004 Free PMC article.
-
Analysis of the kinetic mechanism of recombinant human isoprenylcysteine carboxylmethyltransferase (Icmt).BMC Biochem. 2004 Dec 29;5:19. doi: 10.1186/1471-2091-5-19. BMC Biochem. 2004. PMID: 15625008 Free PMC article.
-
Mutational analysis of the integral membrane methyltransferase isoprenylcysteine carboxyl methyltransferase (ICMT) reveals potential substrate binding sites.J Biol Chem. 2014 Sep 19;289(38):26007-26020. doi: 10.1074/jbc.M114.585125. Epub 2014 Jul 24. J Biol Chem. 2014. PMID: 25059662 Free PMC article.
-
Isoprenyl carboxyl methyltransferase inhibitors: a brief review including recent patents.Amino Acids. 2017 Sep;49(9):1469-1485. doi: 10.1007/s00726-017-2454-x. Epub 2017 Jun 19. Amino Acids. 2017. PMID: 28631011 Free PMC article. Review.
-
Small change, big effect: Taking RAS by the tail through suppression of post-prenylation carboxylmethylation.Small GTPases. 2020 Jul;11(4):271-279. doi: 10.1080/21541248.2017.1415637. Epub 2018 Jan 25. Small GTPases. 2020. PMID: 29261009 Free PMC article. Review.
Cited by
-
Heterologous expression studies of Saccharomyces cerevisiae reveal two distinct trypanosomatid CaaX protease activities and identify their potential targets.Eukaryot Cell. 2009 Dec;8(12):1891-900. doi: 10.1128/EC.00169-09. Epub 2009 Oct 9. Eukaryot Cell. 2009. PMID: 19820121 Free PMC article.
-
Quantitative structure-activity relationship (QSAR) of indoloacetamides as inhibitors of human isoprenylcysteine carboxyl methyltransferase.Bioorg Med Chem Lett. 2007 Feb 15;17(4):1025-32. doi: 10.1016/j.bmcl.2006.11.030. Epub 2006 Nov 15. Bioorg Med Chem Lett. 2007. PMID: 17157012 Free PMC article.
-
Lipid and sulfur substituted prenylcysteine analogs as human Icmt inhibitors.Bioorg Med Chem Lett. 2011 Sep 15;21(18):5616-9. doi: 10.1016/j.bmcl.2011.06.053. Epub 2011 Jun 21. Bioorg Med Chem Lett. 2011. PMID: 21782433 Free PMC article.
-
Evaluation of substrate and inhibitor binding to yeast and human isoprenylcysteine carboxyl methyltransferases (Icmts) using biotinylated benzophenone-containing photoaffinity probes.Biochem Biophys Res Commun. 2012 Jun 22;423(1):98-103. doi: 10.1016/j.bbrc.2012.05.089. Epub 2012 May 23. Biochem Biophys Res Commun. 2012. PMID: 22634004 Free PMC article.
-
Small-molecule inhibitors of the Rce1p CaaX protease.J Biomol Screen. 2007 Oct;12(7):983-93. doi: 10.1177/1087057107307226. J Biomol Screen. 2007. PMID: 17942791 Free PMC article.
References
-
- Zhang FL, Casey PJ. Annu Rev Biochem. 1996;65:241–269. - PubMed
-
- Young SG, Ambroziak P, Kim E, Clarke S. The Enzymes. 3. Vol. 21. Academic Press; San Diego, CA: 2000. pp. 155–213.
-
- Gibbs RA, Zahn TJ, Sebolt-Leopold JS. Curr Med Chem. 2001;8:1437–1466. - PubMed
-
- Hrycyna CA, Clarke S. J Biol Chem. 1992;267:10457–10464. - PubMed
-
- Boyartchuk VL, Ashby MN, Rine J. Science. 1997;275:1796–1800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

