Confocal imaging of [Ca2+] in cellular organelles by SEER, shifted excitation and emission ratioing of fluorescence
- PMID: 15946962
- PMCID: PMC1474212
- DOI: 10.1113/jphysiol.2005.087973
Confocal imaging of [Ca2+] in cellular organelles by SEER, shifted excitation and emission ratioing of fluorescence
Abstract
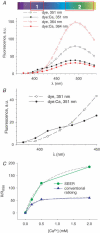
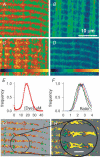
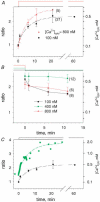
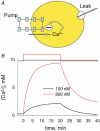
Intracellular calcium signals regulate multiple cellular functions. They depend on release of Ca2+ from cellular stores into the cytosol, a process that appears to be tightly controlled by changes in [Ca2+] within the store. A method to image free [Ca2+] within cellular organelles was devised, which provided the first quantitative confocal images of [Ca2+] inside the sarcoplasmic reticulum (SR) of skeletal muscle. The method exploits, for greater sensitivity, the dual spectral shifts that some fluorescent dyes undergo upon binding Ca2+. It was implemented with mag-indo-1 trapped in the intracellular organelles of frog skeletal muscle and validated showing that it largely monitors [Ca2+] in a caffeine-sensitive compartment with the structure of the SR cisternae. A tentative calibration in situ demonstrated an increase in the dye's dissociation constant, not unlike that observed for other dyes in cellular environments. This increase, together with other characteristics of the ratioing method, placed the half-signal [Ca2+] near 1 mM, a value suitable for cellular stores. Demonstrated advantages of the technique include accuracy (that of a calibrated ratiometric method), dynamic range and sensitivity (from the combination of two spectral shifts), spatial and temporal resolution, and compatibility with a vast array of visible dyes to monitor diverse aspects of cellular function. SEER (shifted excitation and emission ratioing) also provides a [Ca2+]-independent measure of dye concentration in the cell. Store and mitochondrial [Ca2+] ([Ca2+]SR and [Ca2+]mito could be measured separately using the high spatial resolution of SEER. Evolution of [Ca2+]SR was followed upon changes in cytosolic [Ca2+] ([Ca2+]cyto). At [Ca2+]cyto = 100 nM, [Ca2+]mito remained near the lower limit of detection and [Ca2+]SR stabilized at values that were submillimolar according to our tentative calibration. Steady [Ca2+]SR was only slightly higher in 800 nM [Ca2+]cyto, and essentially did not decrease unless [Ca2+]cyto was reduced below 10 nM. While the increase of [Ca2+]SR was limited by loss through Ca2+ release channels, its decrease in low [Ca2+]cyto was largely dependent on leaks through the SR Ca2+ pump.
Figures









Similar articles
-
The changes in Ca2+ sparks associated with measured modifications of intra-store Ca2+ concentration in skeletal muscle.J Gen Physiol. 2006 Jul;128(1):45-54. doi: 10.1085/jgp.200609545. Epub 2006 Jun 12. J Gen Physiol. 2006. PMID: 16769796 Free PMC article.
-
Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle.J Physiol. 2007 Aug 15;583(Pt 1):81-97. doi: 10.1113/jphysiol.2007.135046. Epub 2007 Jun 14. J Physiol. 2007. PMID: 17569733 Free PMC article.
-
Role of calsequestrin evaluated from changes in free and total calcium concentrations in the sarcoplasmic reticulum of frog cut skeletal muscle fibres.J Physiol. 2007 May 15;581(Pt 1):319-67. doi: 10.1113/jphysiol.2006.126474. Epub 2007 Mar 1. J Physiol. 2007. PMID: 17331996 Free PMC article.
-
Calcium uptake and release modulated by counter-ion conductances in the sarcoplasmic reticulum of skeletal muscle.Acta Physiol Scand. 1996 Mar;156(3):387-96. doi: 10.1046/j.1365-201X.1996.212000.x. Acta Physiol Scand. 1996. PMID: 8729699 Review.
-
Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):889-96. doi: 10.1111/j.1440-1681.2007.04708.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645636 Review.
Cited by
-
Voltage clamp methods for the study of membrane currents and SR Ca(2+) release in adult skeletal muscle fibres.Prog Biophys Mol Biol. 2012 Apr;108(3):98-118. doi: 10.1016/j.pbiomolbio.2012.01.001. Epub 2012 Jan 26. Prog Biophys Mol Biol. 2012. PMID: 22306655 Free PMC article. Review.
-
Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the H(V) family.Physiol Rev. 2013 Apr;93(2):599-652. doi: 10.1152/physrev.00011.2012. Physiol Rev. 2013. PMID: 23589829 Free PMC article. Review.
-
Ryanodine receptor luminal Ca2+ regulation: swapping calsequestrin and channel isoforms.Biophys J. 2009 Oct 7;97(7):1961-70. doi: 10.1016/j.bpj.2009.07.030. Biophys J. 2009. PMID: 19804727 Free PMC article.
-
Exploring cells with targeted biosensors.J Gen Physiol. 2017 Jan;149(1):1-36. doi: 10.1085/jgp.201611654. Epub 2016 Dec 27. J Gen Physiol. 2017. PMID: 28028123 Free PMC article. Review.
-
Dynamic measurement of the calcium buffering properties of the sarcoplasmic reticulum in mouse skeletal muscle.J Physiol. 2013 Jan 15;591(2):423-42. doi: 10.1113/jphysiol.2012.243444. Epub 2012 Nov 12. J Physiol. 2013. PMID: 23148320 Free PMC article.
References
-
- Agard DA, Hiraoka Y, Shaw P, Sedat JW. Fluorescence microscopy in three dimensions. Meth Cell Biol. 1989;30:353–377. - PubMed
-
- Art J. Photon detectors for confocal microscopy. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. 2nd. New York: Plenum Press; 1995. pp. 189–190.
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Bevington PR, Robinson DK. Data Reduction and Error Analysis. Boston: Mc Graw Hill; 2003. pp. 43–44.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

