Identification of target genes and a unique cis element regulated by IRF-8 in developing macrophages
- PMID: 15947094
- PMCID: PMC1895144
- DOI: 10.1182/blood-2005-01-0080
Identification of target genes and a unique cis element regulated by IRF-8 in developing macrophages
Abstract
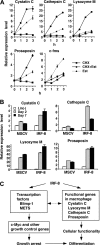
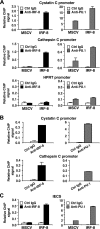
Interferon regulatory factor-8 (IRF-8)/interferon consensus sequence-binding protein (ICSBP) is a transcription factor that controls myeloid-cell development. Microarray gene expression analysis of Irf-8-/- myeloid progenitor cells expressing an IRF-8/estrogen receptor chimera (which differentiate into macrophages after addition of estradiol) was used to identify 69 genes altered by IRF-8 during early differentiation (62 up-regulated and 7 down-regulated). Among them, 4 lysosomal/endosomal enzyme-related genes (cystatin C, cathepsin C, lysozyme, and prosaposin) did not require de novo protein synthesis for induction, suggesting that they were direct targets of IRF-8. We developed a reporter assay system employing a self-inactivating retrovirus and analyzed the cystatin C and cathepsin C promoters. We found that a unique cis element mediates IRF-8-induced activation of both promoters. Similar elements were also found in other IRF-8 target genes with a consensus sequence (GAAANN[N]GGAA) comprising a core IRF-binding motif and an Ets-binding motif; this sequence is similar but distinct from the previously reported Ets/IRF composite element. Chromatin immunoprecipitation assays demonstrated that IRF-8 and the PU.1 Ets transcription factor bind to this element in vivo. Collectively, these data indicate that IRF-8 stimulates transcription of target genes through a novel cis element to specify macrophage differentiation.
Figures




References
-
- Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87: 4025-4039. - PubMed
-
- Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90: 489-519. - PubMed
-
- Tamura T, Ozato K. ICSBP/IRF-8: its regulatory roles in the development of myeloid cells. J Interferon Cytokine Res. 2002;22: 145-152. - PubMed
-
- Holtschke T, Lohler J, Kanno Y, et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87: 307-317. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

