Modulation of glioma BK channels via erbB2
- PMID: 15948146
- PMCID: PMC2548405
- DOI: 10.1002/jnr.20543
Modulation of glioma BK channels via erbB2
Abstract
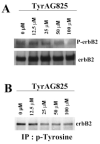
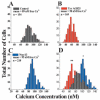
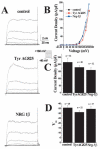
Glioma cells show up-regulation and constitutive activation of erbB2, and its expression correlates positively with increased malignancy. A similar correlation has been demonstrated for the expression of gBK, a calcium-sensitive, large-conductance K(+) channel. We show here that glioma BK channels are a downstream target of erbB2/neuregulin signaling. Tyrphostin AG825 was able to disrupt the constituitive erbB2 activation in a dose-dependent manner, causing a 30-mV positive shift in gBK channel activation in cell-attached patches. Conversely, maximal stimulation of erbB2 with a recombinant neuregulin (NRG-1beta) caused a 12-mV shift in the opposite direction. RT-PCR studies reveal no change in the BK splice variants expressed in treated glioma cells. Furthermore, isolation of surface proteins through biotinylation did not show a change in gBK channel expression, and probing with phospho-specific antibodies showed no alteration in channel phosphorylation. However, fura-II Ca(2+) fluorescence imaging revealed a 35% decrease in the free intracellular Ca(2+) concentration after erbB2 inhibition and an increase in NRG-1beta-treated cells, suggesting that the observed changes most likely were due to alterations in [Ca(2+)](i). Consistent with this conclusion, neither tyrphostin AG825 nor NRG-1beta was able to modulate gBK channels under inside-out or whole-cell recording conditions when intracellular Ca(2+) was fixed. Thus, gBK channels are a downstream target for the abundantly expressed neuregulin-1 receptor erbB2 in glioma cells. However, unlike the case in other systems, this modulation appears to occur via changes in [Ca(2+)](i) without changes in channel expression or phosphorylation. The enhanced sensitivity of gBK channels in glioma cells to small, physiological Ca(2+) changes appears to be a prerequisite for this modulation.
Figures





Similar articles
-
Enhancement effects of martentoxin on glioma BK channel and BK channel (α+β1) subtypes.PLoS One. 2011 Mar 18;6(3):e15896. doi: 10.1371/journal.pone.0015896. PLoS One. 2011. PMID: 21445248 Free PMC article.
-
Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells.J Neurosci Res. 2004 Oct 15;78(2):224-34. doi: 10.1002/jnr.20240. J Neurosci Res. 2004. PMID: 15378515 Free PMC article.
-
Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells.J Neurosci. 2002 Mar 1;22(5):1840-9. doi: 10.1523/JNEUROSCI.22-05-01840.2002. J Neurosci. 2002. PMID: 11880513 Free PMC article.
-
BK channels in human glioma cells have enhanced calcium sensitivity.Glia. 2002 Jun;38(4):281-91. doi: 10.1002/glia.10064. Glia. 2002. PMID: 12007141
-
Large-conductance Ca2+- activated K+ channels:physiological role and pharmacology.Curr Med Chem. 2003 Apr;10(8):649-61. doi: 10.2174/0929867033457863. Curr Med Chem. 2003. PMID: 12678784 Review.
Cited by
-
Targeted brain tumor treatment-current perspectives.Drug Target Insights. 2007;2:197-207. Epub 2007 Aug 17. Drug Target Insights. 2007. PMID: 21901074 Free PMC article.
-
BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts: a novel mechanism for coupling [Ca(2+)](i) to ion channel activation.J Biol Chem. 2007 Oct 26;282(43):31558-68. doi: 10.1074/jbc.M702866200. Epub 2007 Aug 21. J Biol Chem. 2007. PMID: 17711864 Free PMC article.
-
Enhancement effects of martentoxin on glioma BK channel and BK channel (α+β1) subtypes.PLoS One. 2011 Mar 18;6(3):e15896. doi: 10.1371/journal.pone.0015896. PLoS One. 2011. PMID: 21445248 Free PMC article.
-
Functional upregulation of Ca(2+)-activated K(+) channels in the development of substantia nigra dopamine neurons.PLoS One. 2012;7(12):e51610. doi: 10.1371/journal.pone.0051610. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284723 Free PMC article.
References
-
- Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. - PubMed
-
- Basrai D, Kraft R, Bollensdorff C, Liebmann L, Benndorf K, Patt S. BK channel blockers inhibit potassium-induced proliferation of human astrocytoma cells. Neuroreport. 2002;13:403–407. - PubMed
-
- Bordey A, Ullrich N, Sontheimer H. Electrophysiological characterization of astrocytes and astrocytoma cells in slices from human biopsies and experimental intracranial tumors. Soc Neurosci Abstr. 1996;22:594.13.
-
- Bordey A, Sontheimer H, Trouslard J. Muscarinic activation of BK channels induces membrane oscillations in glioma cells and leads to inhibition of cell migration. J Membrane Biol. 2000;176:31–40. - PubMed
-
- Bringmann A, Biedermann B, Reichenbach A. Expression of potassium channels during postnatal differentiation of rabbit Muller glial cells. Eur J Neurosci. 1999;11:2883–2896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

