How children's responses to drugs differ from adults
- PMID: 15948930
- PMCID: PMC1884865
- DOI: 10.1111/j.1365-2125.2005.02445.x
How children's responses to drugs differ from adults
Abstract
Children are not small adults. However, the main thesis of this review will be that children's responses to drugs have much in common with the responses in adults and indeed in other mammals. Often, it is assumed that drug effects differ in children but in reality this perception often arises because the drugs have not been adequately studied in paediatric populations of different ages and with different diseases. There may also be difficulties in measuring small but significant effects because the outcome measures are more difficult to assess in children. In some cases, stage of development can alter the action of, and response to, a drug - a truly age-dependent difference in pharmacodynamics. This may be true of both the desired action and adverse events. Examples are given. Programming by drugs is also a phenomenon almost exclusive to early life, i.e. permanent effects result from a stimulus applied at a sensitive point in development ('critical window'), often in fetal or neonatal life. Again, examples are discussed. Different pathophysiology, different disease variants, different pharmacodynamics, different 'host' response and different adverse drug reactions can all explain why some drugs behave differently in children. However, we need to explore ways to avoid re-inventing the wheel by determining how data from adult animal and human models can help inform research and practice for children.
Figures
References
-
- Takahashi H, Ishikawa S, Nomoto S, Nishigaki Y, Ando F, Kashima T, Kimura S, Kanamori M, Echizen H. Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin Pharmacol Ther. 2000;68:541–55. - PubMed
-
- Marshall JD, Kearns GL. Developmental pharmacodynamics of cyclosporine. Clin Pharmacol Ther. 1999;66:66–75. - PubMed
-
- MacKenzie C. Effects of inhaled corticosteroids on growth. J Allergy Clin Immunol. 1998;101:451–5. - PubMed
-
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064–9. - PubMed
-
- Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002;16:1017–26. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

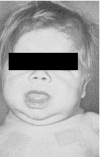
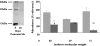
 ), 28 days (
), 28 days ( )
)