Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development
- PMID: 15951419
- PMCID: PMC1157054
- DOI: 10.1073/pnas.0503741102
Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development
Abstract
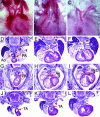
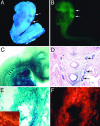
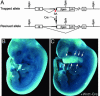
Members of the myocardin-related family of transcription factors play critical roles in regulating vascular smooth muscle and cardiac differentiation. To examine the function of myocardin-related transcription factor (MRTF)-B, mice were generated from ES cells harboring a conditional insertional mutation, or gene trap, of the MRTF-B gene. Expression of the MRTF-B mutant allele results in a fusion protein consisting of the N terminus of MRTF-B fused to beta-galactosidase, which is functionally null. Homozygous MRTF-B gene trap mice (MRTF-B-/-) die between embryonic day (E)17.5 and postnatal day 1 from cardiac outflow tract defects. MRTF-B is expressed in the premigratory neural crest, in rhombomeres 3 and 5, and in the neural crest-derived mesenchyme surrounding the aortic arch arteries. Consistent with the pattern of expression, E10.5 and E11.5 MRTF-B-/- mutants exhibit deformation of aortic arch arteries 3, 4, and 6 and severe attenuation of smooth muscle cell differentiation in the arch arteries and the aorticopulmonary septum, despite normal migration and initial patterning of cardiac neural crest cells. Remarkably, the observed pathology was rescued and viable mice generated by intercrossing MRTF-B mutants with mice expressing Cre recombinase under the transcriptional control of the neural crest-restricted Wnt-1 promoter, which results in restoration of normal MRTF-B expression in the neural crest. Taken together, these studies reveal that MRTF-B plays a critical role in regulating differentiation of cardiac neural crest cells into smooth muscle and demonstrate that neural crest-derived smooth muscle differentiation is specifically required for normal cardiovascular morphogenesis.
Figures
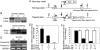

References
-
- Kirby, M. L., Gale, T. F. & Stewart, D. E. (1983) Science 220, 1059–1061. - PubMed
-
- Creazzo, T. L., Godt, R. E., Leatherbury, L., Conway, S. J. & Kirby, M. L. (1998) Annu. Rev. Physiol. 60, 267–286. - PubMed
-
- Jiang, X., Rowitch, D. H., Soriano, P., McMahon, A. P. & Sucov, H. M. (2000) Development (Cambridge, U.K.) 127, 1607–1616. - PubMed
-
- Li, J., Chen, F. & Epstein, J. A. (2000) Genesis 26, 162–164. - PubMed
-
- Gruber, P. J. & Epstein, J. A. (2004) Circ. Res. 94, 273–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

