Pectin methylesterase, a regulator of pollen tube growth
- PMID: 15951488
- PMCID: PMC1176407
- DOI: 10.1104/pp.105.059865
Pectin methylesterase, a regulator of pollen tube growth
Abstract
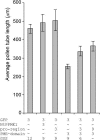
The apical wall of growing pollen tubes must be strong enough to withstand the internal turgor pressure, but plastic enough to allow the incorporation of new membrane and cell wall material to support polarized tip growth. These essential rheological properties appear to be controlled by pectins, which constitute the principal component of the apical cell wall. Pectins are secreted as methylesters and subsequently deesterified by the enzyme pectin methylesterase (PME) in a process that exposes acidic residues. These carboxyls can be cross-linked by calcium, which structurally rigidifies the cell wall. Here, we examine the role of PME in cell elongation and the regulation of its secretion and enzymatic activity. Application of an exogenous PME induces thickening of the apical cell wall and inhibits pollen tube growth. Screening a Nicotiana tabacum pollen cDNA library yielded a pollen-specific PME, NtPPME1, containing a pre-region and a pro-region. Expression studies with green fluorescent protein fusion proteins show that the pro-region participates in the correct targeting of the mature PME. Results from in vitro growth analysis and immunolocalization studies using antipectin antibodies (JIM5 and JIM7) provide support for the idea that the pro-region acts as an intracellular inhibitor of PME activity, thereby preventing premature deesterification of pectins. In addition to providing experimental data that help resolve the significance and function of the pro-region, our results give insight into the mechanism by which PME and its pro-region regulate the cell wall dynamics of growing pollen tubes.
Figures




References
-
- Albani D, Altosaar I, Arnison PG, Fabijanski SF (1991) A gene showing sequence similarity to pectin esterase is specifically expressed in developing pollen of Brassica napus. Sequences in its 5′ flanking region are conserved in other pollen-specific promoters. Plant Mol Biol 16: 501–513 - PubMed
-
- Balestrieri C, Castaldo D, Giovane A, Quagliuolo L, Servillo L (1990) A glycoprotein inhibitor of pectin methylesterase in kiwi fruit (Actinidia chinensis). Eur J Biochem 193: 183–187 - PubMed
-
- Bordenave M (1996) Analysis of pectin methyl esterases. In HF Linskens, JF Jackson, eds, Plant Cell Wall Analysis, Vol 17. Springer-Verlag, Berlin, pp 165–180
-
- Brandizzi F, Irons SL, Johansen J, Kotzer A, Neumann U (2004) GFP is the way to glow: bioimaging of the plant endomembrane system. J Microsc 214: 138–158 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

