Endosome-to-cytosol transport of viral nucleocapsids
- PMID: 15951806
- PMCID: PMC3360589
- DOI: 10.1038/ncb1269
Endosome-to-cytosol transport of viral nucleocapsids
Abstract
During viral infection, fusion of the viral envelope with endosomal membranes and nucleocapsid release were thought to be concomitant events. We show here that for the vesicular stomatitis virus they occur sequentially, at two successive steps of the endocytic pathway. Fusion already occurs in transport intermediates between early and late endosomes, presumably releasing the nucleocapsid within the lumen of intra-endosomal vesicles, where it remains hidden. Transport to late endosomes is then required for the nucleocapsid to be delivered to the cytoplasm. This last step, which initiates infection, depends on the late endosomal lipid lysobisphosphatidic acid (LBPA) and its putative effector Alix/AIP1, and is regulated by phosphatidylinositol-3-phosphate (PtdIns3P) signalling via the PtdIns3P-binding protein Snx16. We conclude that the nucleocapsid is exported into the cytoplasm after the back-fusion of internal vesicles with the limiting membrane of late endosomes, and that this process is controlled by the phospholipids LBPA and PtdIns3P and their effectors.
Figures
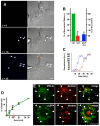

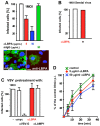


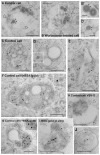

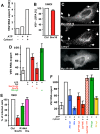
Comment in
-
Viral entry: a detour through multivesicular bodies.Nat Cell Biol. 2005 Jul;7(7):641-2. doi: 10.1038/ncb0705-641. Nat Cell Biol. 2005. PMID: 15990891 No abstract available.
References
-
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
-
- Mukherjee S, Maxfield FR. Role of Membrane Organization and Membrane Domains in Endocytic Lipid Trafficking. Traffic. 2000;1:203–211. - PubMed
-
- Sannerud R, Saraste J, Goud B. Retrograde traffic in the biosynthetic-secretory route: pathways and machinery. Curr Opin Cell Biol. 2003;15:438–45. - PubMed
-
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nature Reviews Molecular Cell Biology. 2001;2:721–30. - PubMed
-
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

