Therapeutic targeting of CCR1 attenuates established chronic fungal asthma in mice
- PMID: 15951834
- PMCID: PMC1576221
- DOI: 10.1038/sj.bjp.0706243
Therapeutic targeting of CCR1 attenuates established chronic fungal asthma in mice
Abstract
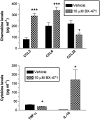
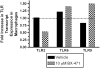
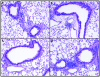
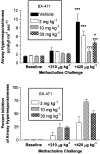
CC chemokine receptor 1 (CCR1) represents a promising target in chronic airway inflammation and remodeling due to fungus-associated allergic asthma. The present study addressed the therapeutic effect of a nonpeptide CCR1 antagonist, BX-471, in a model of chronic fungal asthma induced by Aspergillus fumigatus conidia. BX-471 treatment of isolated macrophages inhibited CCL22 and TNF-alpha and promoted IL-10 release. BX-471 also increased toll like receptor-9 (TLR9) and decreased TLR2 and TLR6 expression in these cells. When administered daily by intraperitoneal injection, from days 15 to 30 after the initiation of chronic fungal asthma, BX-471 (3, 10, or 30 mg kg(-1)) dose-dependently reduced airway inflammation, hyper-responsiveness, and remodeling at day 30 after conidia challenge. The maximal therapeutic effect was observed at the 10 mg kg(-1) dose. In summary, the therapeutic administration of BX-471 significantly attenuated experimental fungal asthma via its effects on both innate and adaptive immune processes.
Figures





References
-
- ANDERS H.J., BANAS B., LINDE Y., WELLER L., COHEN C.D., KRETZLER M., MARTIN S., VIELHAUER V., SCHLONDORFF D., GRONE H.J. Bacterial CpG-DNA aggravates immune complex glomerulonephritis: role of TLR9-mediated expression of chemokines and chemokine receptors. J. Am. Soc. Nephrol. 2003;14:317–326. - PubMed
-
- ANDERS H.J., BELEMEZOVA E., EIS V., SEGERER S., VIELHAUER V., PEREZ DE LEMA G., KRETZLER M., COHEN C.D., FRINK M., HORUK R., HUDKINS K.L., ALPERS C.E., MAMPASO F., SCHLONDORFF D. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas (lpr) mice. J. Am. Soc. Nephrol. 2004;15:1504–1513. - PubMed
-
- ANDERS H.J., VIELHAUER V., FRINK M., LINDE Y., COHEN C.D., BLATTNER S.M., KRETZLER M., STRUTZ F., MACK M., GRONE H.J., ONUFFER J., HORUK R., NELSON P.J., SCHLONDORFF D. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J. Clin. Invest. 2002;109:251–259. - PMC - PubMed
-
- BACHAR O., ADNER M., UDDMAN R., CARDELL L.O. Toll-like receptor stimulation induces airway hyper-responsiveness to bradykinin, an effect mediated by JNK and NF-kappa B signaling pathways. Eur. J. Immunol. 2004;34:1196–1207. - PubMed
-
- BARNES P.J. Cytokine-directed therapies for asthma. J. Allergy Clin. Immunol. 2001;108:S72–S76. - PubMed

